六配位R2SnCl2(N⌒N)型新配合物的合成及其体外抑癌活性*
作者:李青山 黄计军 杨 频 王瑞卿
单位:李青山 黄计军 山西医科大学药学系,太原 030001;杨 频 王瑞卿 山西大学分子所,太原 030001
关键词:有机锡化合物;合成;抗癌活性
SynthesisSynthesis,Characterization
and Their In Vitro Antitumor Activity of Di-n-Butyl
(or Phenyl)Dichloride Complexes with N⌒N Type Ligands
Li Qingshan,Huang Jijun
, 百拇医药 (Department of Pharmaceutical Science,Shanxi Medical University,Taiyuan,030001)
Yang Pin,Wang Ruiqing
(Institute of Molecular Science,Shanxi University,Taiyuan 030001)
Abstract Four new diorganotin complexes were synthesized and characterized by IR and 1H NMR.Their in vitro antitumor activities against a series of human tumor cell lines such as P388tumor cells were tested.The results showed that the complexes mainly exhibited strong antitumor activities against P388,but presented no activities against other tumor cell lines.The structure-activity relationships were discussed.
, http://www.100md.com
Key words diorganotin complex;synthesis;antitumor activity
从80年代初起,有机锡化合物的抗癌活性日益受到重视,研究人员合成了大量的有机锡化合物,并对其进行了较全面的抗癌活性筛选〔1~3〕.
吡唑衍生物具有抗菌〔4,5〕和杀虫作用〔6〕,其结构中有多个配位点,本文合成了两个新的吡唑衍生物(见图1)并用其作为双氮螯合配体来合成数个六配位R2SnCl2(N⌒N)型新配合物,并对它们的结构进行了表征,考察了它们对多种癌细胞的抑制作用,探讨了构效关系,结果表明这些六配位R2SnCl2(N⌒N)型新配合物主要对P388淋巴白血病有较强的抑制作用.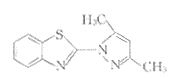
, http://www.100md.com
Ⅰ〔N⌒N(1)〕
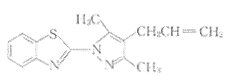
Ⅱ〔N⌒N(2)〕
Fig.1 Structures of the new ligands
配体的合成
配体I〔N⌒N(1)〕的合成:称取3.4 g(0.02 mol)2-巯基苯并噻唑基肼,放入装有回流冷凝管的50 mL的圆底烧瓶中,然后加入6 mL(0.06 mol)乙酰丙酮和16 mL无水乙醇,在90℃回流7 h,冷却,抽滤,用无水乙醇重结晶,得絮状结晶,真空干燥,mp 148.3~148.7 ℃.元素分析,实验值(%):C 62.98,H 4.86,N 18.51;理论值(%):C 62.85,H 4.84,N 18.33.IR(KBr) cm-1:1600(m,C=N,吡唑),1530(m,C=N,苯并噻唑),1570(m,C=C),1026(w,N=N).1H-NMR(CDCl3)δ:2.38(s,3H,CH3—R),2.77(s,3H,CH3—R),6.02(s,1H,C=H),7.35~7.77(m,4H,-Ph).
cm-1:1600(m,C=N,吡唑),1530(m,C=N,苯并噻唑),1570(m,C=C),1026(w,N=N).1H-NMR(CDCl3)δ:2.38(s,3H,CH3—R),2.77(s,3H,CH3—R),6.02(s,1H,C=H),7.35~7.77(m,4H,-Ph).
, 百拇医药
配体Ⅱ〔N⌒N(2)〕的合成:称取3.5 g(0.02 mol)2-巯基苯并噻唑基肼,放入装有回流冷凝管的50 mL的圆底烧瓶中,加入6 mL(0.06 mol)3-烯丙基-2,4-戊二酮和16 mL无水乙醇,于85℃回流5 h,冷却,瓶内有淡黄色的针状结晶析出,用95%乙醇重结晶,真空干燥,mp 131.0~132.0℃.元素分析,实验值(%):C 66.82,H 5.59,N 15.77;理论值(%):C 67.12,H 5.27,N 15.66.IR(KBr) cm-1:1607(m,C=N,吡唑),1533(m,C=N,苯并噻唑),1579(m,C=C),1018(w,N=N).1H-NMR(CDCl3)δ:2.25(s,3H,CH3—R),2.71(s,3H,CH3—R),3.31(b,2H,R-CH2-R),5.14(m,2H,R=CH2),5.66(m,1H,R-CH=R),7.36~7.82(m,4H,-Ph ).
cm-1:1607(m,C=N,吡唑),1533(m,C=N,苯并噻唑),1579(m,C=C),1018(w,N=N).1H-NMR(CDCl3)δ:2.25(s,3H,CH3—R),2.71(s,3H,CH3—R),3.31(b,2H,R-CH2-R),5.14(m,2H,R=CH2),5.66(m,1H,R-CH=R),7.36~7.82(m,4H,-Ph ).
, 百拇医药
配合物的合成
将R2SnCl2与配体按1∶1的摩尔质量比在干燥的苯中加热回流2 h,然后冷却,在空气中缓慢挥发溶剂,二苯基锡配合物可得到白色晶体,二正丁基锡配合物得到浅灰色粉末.元素分析与计算值完全吻合(见表1),表明产品为预期产物,结构见图2.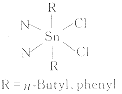
Fig.2 Structures of the complexes
Tab.1 Physical data and IR data for the diorganotin complexes
Complexes
, http://www.100md.com
Elemental analysis/%
Found(Calc.)
mp/℃
ν Sn-C
ν Sn-Cl
ν Sn-N
C
H
N
(n-Bu)2SnCl2[N⌒N(1)]
45.05
, 百拇医药 5.44
7.88
114.5~114.9
508 w,531 m
221 s,248 s
440 m
(45.53)
(5.46)
(7.95)
Ph2SnCl2[N⌒N(1)]
50.27
3.67
, 百拇医药
7.33
126.3~127.2
488 w,567 m
220 s,245 s
437 m
(50.64)
(3.42)
(7.21)
(n-Bu)2SnCl2[N⌒N(2)]
69.14
6.91
, 百拇医药
7.33
97.9~98.6
478 vw,595 m
229 s,272 s
456 s
(69.28)
(6.85)
(7.40)
Ph2SnCl2[N⌒N(2)]
52.88
4.08
, 百拇医药 6.85
104.5~105.2
534 w,559 m
227 s,280 m
427 s
(52.67)
(4.03)
(6.98)
(n-Bu)2SnCl2
517 m,602 s
340 s,356 s
, 百拇医药
Ph2SnCl2
441 m,565 m
319 s,348 s
结构表征和抗癌活性
红外光谱表明,配合物在430 cm-1附近有一中强吸收峰,这是Sn与配体氮原子结合所产生的特征Sn-N峰,同时原有Sn-C,SnCl 键都有不同程度的减弱,说明Sn与配体N位点键合.各配合物中都有两个Sn-C键,说明烃基处于畸变八面体的反位〔7〕.
配体与Sn键合后各质子的1H-NMR化学位移没有明显变化(见表2).可能是由于配合物在有机溶剂中发生分解而成为原料所致〔8〕.另外也可能是因为这些质子与配位点离得较远,因而配位作用对其化学位移影响不大.Tab.2 1H-NMR data for the diorganotin complexes①
, 百拇医药
Complexes
Ligands
RSn
CH3-R
CH3-R
=CH-
-Ph
R-CH2-R
N⌒N(1)
2.38
2.77
6.02
, http://www.100md.com
7.35~7.77
(s,3H)
(s,3H)
(s,1H)
(m,4H)
(n-Bu)2SnCl2
2.38
2.77
6.03
7.35~7.77
0.86~1.55
-[N⌒N(1)]
, http://www.100md.com
(s,3H)
(s,3H)
(s,1H)
(m,4H)
(m,18H)
Ph2SnCl2-
2.38
2.77
6.04
7.36~7.78
7.68~7.78
[N⌒N(1)]
, http://www.100md.com
(s,3H)
(s,3H)
(s,1H)
(m,4H)
(m,10H)
N⌒N(1)
2.25
2.71
5.15,5.66
7.36~7.82
3.31
(s,3H)
, 百拇医药
(s,3H)
(m,2H),(m,1H)
(m,4H)
(br,2H)
(n-Bu)2SnCl2-
2.25
2.71
5.14,5.66
7.35~7.83
3.31
0.85~1.54
, 百拇医药 [N⌒N(2)]
(s,3H)
(s,3H)
(m,2H),(m,1H)
(m,4H)
(br,2H)
(m,18H)
Ph2SnCl2-
2.25
2.71
5.15,5.67
7.36~7.83
, 百拇医药
3.31
7.58~7.76
[N⌒N(2)]
(s,3H)
(s,3H)
(m,2H),(m,1H)
(m,4H)
(br,2H)
(m,10H)
①1H-NMR was recorded in CDCl3 with TMS as an internal standard
, http://www.100md.com
从表3可见,配体对P388,A549和SGC等癌细胞均无抑制作用,而各配合物对P388显示了很强的活性.除二正丁基锡配合物对A549和SGC有较弱的抑制外,其它配合物对A549和SGC无抗癌活性,与许多文献结果相似,此类型配合物主要是对P388淋巴白血病有活性〔9〕.从表中还可看出,二正丁基配合物的活性明显高于二苯基锡配合物.
Tab.3 In vitro antitumor activity of diorganotin complexes (mol/L)
Complexes
P388
A549
, http://www.100md.com
SGC
100
10
1
100
10
1
100
10
1
[N⌒N(1)]
inactive
inactive
, http://www.100md.com
inactive
(n-Bu)2SnCl2[N⌒N(1)]
98.5
97.0
23.1
96.2
95.1
7.1
84.6
82.0
0
Ph2SnCl2[N⌒N(1)]
, 百拇医药
100
96.4
0.9
inactive
inactive
[N⌒N(2)]
inactive
inactive
inactive
(n-Bu)2SnCl2[N⌒N(2)]
100
97.3
, http://www.100md.com
96.5
93.6
57.0
33.0
89.8
8.3
0
Ph2SnCl2[N⌒N(1)]
96.4
87.5
51.9
59.6
, http://www.100md.com
0
0
81.5
43.5
0
*国家科技部重点资助项目(No.96-901-06-038)和国家自然科学基金资助项目(No.39900185)
作者简介:李青山 通讯联系人
王瑞卿 北京医科大学天然药物及仿生药物国家重点实验室,北京 100083
参考文献
1 Narayan VL,Reinhoudt DN.Structure-activity relationships of antitumor agents.The Hague:Martinus Ni Jhoft,1983.5~197
, http://www.100md.com
2 Gielen M.Tin based antitumor drugs.Berlin:Springer Verlag,1990.1~126
3 胡春,谭日红.抗癌活性有机锡化合物的研究进展.中国药学杂志,1992,27(8):455~457
4 Arali VH,Revankar VK,Mahale VB.Synthesis,characterization and biological studies of 2-{3,5-dimethylpyraole-1-y}benzothiazole complexes of cobalt(Ⅱ),nickel(Ⅱ) and copper(Ⅱ).Transition Met Chem,1993,18(1):158~162
5 Revankar VK,Arail VH,Mahale VB.Synthesis,charactderization and biological studies of 3-acetylcoumarin hydrazones with cobalt(Ⅱ),nickel(Ⅱ) and coper(Ⅱ).Indian J Chem,1990,29A:889~893
, http://www.100md.com
6 Hoffman H,Hamman I.Insecticidal N,N-dimethyl-O-[1-methyl-3-N-methyl-carbaminyl-methylpyrazole(5)yl]carbaminic acid ester.Ger Offen.1975,420(2):2360~2362
7 Sandhu GK,Boparoy.Diorganotin derivatives of thiophene 2-carboxylic acid.J Organomet Chem,1991,111(1):89~94
8 Pettinari C,Pellei M,Marchetti F,et al.Tin and Organotin complexes containing mono or bidentate N-donor ligands.Polyhedron,1998,17(4):561~576
9 Saxena A,Huber F.Organotin compounds and cancer chemotherapy.Coord Chem Rev,1989,95(1):109~123
收稿日期:1999-08-15, http://www.100md.com
单位:李青山 黄计军 山西医科大学药学系,太原 030001;杨 频 王瑞卿 山西大学分子所,太原 030001
关键词:有机锡化合物;合成;抗癌活性
SynthesisSynthesis,Characterization
and Their In Vitro Antitumor Activity of Di-n-Butyl
(or Phenyl)Dichloride Complexes with N⌒N Type Ligands
Li Qingshan,Huang Jijun
, 百拇医药 (Department of Pharmaceutical Science,Shanxi Medical University,Taiyuan,030001)
Yang Pin,Wang Ruiqing
(Institute of Molecular Science,Shanxi University,Taiyuan 030001)
Abstract Four new diorganotin complexes were synthesized and characterized by IR and 1H NMR.Their in vitro antitumor activities against a series of human tumor cell lines such as P388tumor cells were tested.The results showed that the complexes mainly exhibited strong antitumor activities against P388,but presented no activities against other tumor cell lines.The structure-activity relationships were discussed.
, http://www.100md.com
Key words diorganotin complex;synthesis;antitumor activity
从80年代初起,有机锡化合物的抗癌活性日益受到重视,研究人员合成了大量的有机锡化合物,并对其进行了较全面的抗癌活性筛选〔1~3〕.
吡唑衍生物具有抗菌〔4,5〕和杀虫作用〔6〕,其结构中有多个配位点,本文合成了两个新的吡唑衍生物(见图1)并用其作为双氮螯合配体来合成数个六配位R2SnCl2(N⌒N)型新配合物,并对它们的结构进行了表征,考察了它们对多种癌细胞的抑制作用,探讨了构效关系,结果表明这些六配位R2SnCl2(N⌒N)型新配合物主要对P388淋巴白血病有较强的抑制作用.


, http://www.100md.com
Ⅰ〔N⌒N(1)〕
Ⅱ〔N⌒N(2)〕
Fig.1 Structures of the new ligands
配体的合成
配体I〔N⌒N(1)〕的合成:称取3.4 g(0.02 mol)2-巯基苯并噻唑基肼,放入装有回流冷凝管的50 mL的圆底烧瓶中,然后加入6 mL(0.06 mol)乙酰丙酮和16 mL无水乙醇,在90℃回流7 h,冷却,抽滤,用无水乙醇重结晶,得絮状结晶,真空干燥,mp 148.3~148.7 ℃.元素分析,实验值(%):C 62.98,H 4.86,N 18.51;理论值(%):C 62.85,H 4.84,N 18.33.IR(KBr)
, 百拇医药
配体Ⅱ〔N⌒N(2)〕的合成:称取3.5 g(0.02 mol)2-巯基苯并噻唑基肼,放入装有回流冷凝管的50 mL的圆底烧瓶中,加入6 mL(0.06 mol)3-烯丙基-2,4-戊二酮和16 mL无水乙醇,于85℃回流5 h,冷却,瓶内有淡黄色的针状结晶析出,用95%乙醇重结晶,真空干燥,mp 131.0~132.0℃.元素分析,实验值(%):C 66.82,H 5.59,N 15.77;理论值(%):C 67.12,H 5.27,N 15.66.IR(KBr)
, 百拇医药
配合物的合成
将R2SnCl2与配体按1∶1的摩尔质量比在干燥的苯中加热回流2 h,然后冷却,在空气中缓慢挥发溶剂,二苯基锡配合物可得到白色晶体,二正丁基锡配合物得到浅灰色粉末.元素分析与计算值完全吻合(见表1),表明产品为预期产物,结构见图2.
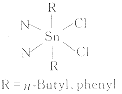
Fig.2 Structures of the complexes
Tab.1 Physical data and IR data for the diorganotin complexes
Complexes
, http://www.100md.com
Elemental analysis/%
Found(Calc.)
mp/℃
ν Sn-C
ν Sn-Cl
ν Sn-N
C
H
N
(n-Bu)2SnCl2[N⌒N(1)]
45.05
, 百拇医药 5.44
7.88
114.5~114.9
508 w,531 m
221 s,248 s
440 m
(45.53)
(5.46)
(7.95)
Ph2SnCl2[N⌒N(1)]
50.27
3.67
, 百拇医药
7.33
126.3~127.2
488 w,567 m
220 s,245 s
437 m
(50.64)
(3.42)
(7.21)
(n-Bu)2SnCl2[N⌒N(2)]
69.14
6.91
, 百拇医药
7.33
97.9~98.6
478 vw,595 m
229 s,272 s
456 s
(69.28)
(6.85)
(7.40)
Ph2SnCl2[N⌒N(2)]
52.88
4.08
, 百拇医药 6.85
104.5~105.2
534 w,559 m
227 s,280 m
427 s
(52.67)
(4.03)
(6.98)
(n-Bu)2SnCl2
517 m,602 s
340 s,356 s
, 百拇医药
Ph2SnCl2
441 m,565 m
319 s,348 s
结构表征和抗癌活性
红外光谱表明,配合物在430 cm-1附近有一中强吸收峰,这是Sn与配体氮原子结合所产生的特征Sn-N峰,同时原有Sn-C,SnCl 键都有不同程度的减弱,说明Sn与配体N位点键合.各配合物中都有两个Sn-C键,说明烃基处于畸变八面体的反位〔7〕.
配体与Sn键合后各质子的1H-NMR化学位移没有明显变化(见表2).可能是由于配合物在有机溶剂中发生分解而成为原料所致〔8〕.另外也可能是因为这些质子与配位点离得较远,因而配位作用对其化学位移影响不大.Tab.2 1H-NMR data for the diorganotin complexes①
, 百拇医药
Complexes
Ligands
RSn
CH3-R
CH3-R
=CH-
-Ph
R-CH2-R
N⌒N(1)
2.38
2.77
6.02
, http://www.100md.com
7.35~7.77
(s,3H)
(s,3H)
(s,1H)
(m,4H)
(n-Bu)2SnCl2
2.38
2.77
6.03
7.35~7.77
0.86~1.55
-[N⌒N(1)]
, http://www.100md.com
(s,3H)
(s,3H)
(s,1H)
(m,4H)
(m,18H)
Ph2SnCl2-
2.38
2.77
6.04
7.36~7.78
7.68~7.78
[N⌒N(1)]
, http://www.100md.com
(s,3H)
(s,3H)
(s,1H)
(m,4H)
(m,10H)
N⌒N(1)
2.25
2.71
5.15,5.66
7.36~7.82
3.31
(s,3H)
, 百拇医药
(s,3H)
(m,2H),(m,1H)
(m,4H)
(br,2H)
(n-Bu)2SnCl2-
2.25
2.71
5.14,5.66
7.35~7.83
3.31
0.85~1.54
, 百拇医药 [N⌒N(2)]
(s,3H)
(s,3H)
(m,2H),(m,1H)
(m,4H)
(br,2H)
(m,18H)
Ph2SnCl2-
2.25
2.71
5.15,5.67
7.36~7.83
, 百拇医药
3.31
7.58~7.76
[N⌒N(2)]
(s,3H)
(s,3H)
(m,2H),(m,1H)
(m,4H)
(br,2H)
(m,10H)
①1H-NMR was recorded in CDCl3 with TMS as an internal standard
, http://www.100md.com
从表3可见,配体对P388,A549和SGC等癌细胞均无抑制作用,而各配合物对P388显示了很强的活性.除二正丁基锡配合物对A549和SGC有较弱的抑制外,其它配合物对A549和SGC无抗癌活性,与许多文献结果相似,此类型配合物主要是对P388淋巴白血病有活性〔9〕.从表中还可看出,二正丁基配合物的活性明显高于二苯基锡配合物.
Tab.3 In vitro antitumor activity of diorganotin complexes (mol/L)
Complexes
P388
A549
, http://www.100md.com
SGC
100
10
1
100
10
1
100
10
1
[N⌒N(1)]
inactive
inactive
, http://www.100md.com
inactive
(n-Bu)2SnCl2[N⌒N(1)]
98.5
97.0
23.1
96.2
95.1
7.1
84.6
82.0
0
Ph2SnCl2[N⌒N(1)]
, 百拇医药
100
96.4
0.9
inactive
inactive
[N⌒N(2)]
inactive
inactive
inactive
(n-Bu)2SnCl2[N⌒N(2)]
100
97.3
, http://www.100md.com
96.5
93.6
57.0
33.0
89.8
8.3
0
Ph2SnCl2[N⌒N(1)]
96.4
87.5
51.9
59.6
, http://www.100md.com
0
0
81.5
43.5
0
*国家科技部重点资助项目(No.96-901-06-038)和国家自然科学基金资助项目(No.39900185)
作者简介:李青山 通讯联系人
王瑞卿 北京医科大学天然药物及仿生药物国家重点实验室,北京 100083
参考文献
1 Narayan VL,Reinhoudt DN.Structure-activity relationships of antitumor agents.The Hague:Martinus Ni Jhoft,1983.5~197
, http://www.100md.com
2 Gielen M.Tin based antitumor drugs.Berlin:Springer Verlag,1990.1~126
3 胡春,谭日红.抗癌活性有机锡化合物的研究进展.中国药学杂志,1992,27(8):455~457
4 Arali VH,Revankar VK,Mahale VB.Synthesis,characterization and biological studies of 2-{3,5-dimethylpyraole-1-y}benzothiazole complexes of cobalt(Ⅱ),nickel(Ⅱ) and copper(Ⅱ).Transition Met Chem,1993,18(1):158~162
5 Revankar VK,Arail VH,Mahale VB.Synthesis,charactderization and biological studies of 3-acetylcoumarin hydrazones with cobalt(Ⅱ),nickel(Ⅱ) and coper(Ⅱ).Indian J Chem,1990,29A:889~893
, http://www.100md.com
6 Hoffman H,Hamman I.Insecticidal N,N-dimethyl-O-[1-methyl-3-N-methyl-carbaminyl-methylpyrazole(5)yl]carbaminic acid ester.Ger Offen.1975,420(2):2360~2362
7 Sandhu GK,Boparoy.Diorganotin derivatives of thiophene 2-carboxylic acid.J Organomet Chem,1991,111(1):89~94
8 Pettinari C,Pellei M,Marchetti F,et al.Tin and Organotin complexes containing mono or bidentate N-donor ligands.Polyhedron,1998,17(4):561~576
9 Saxena A,Huber F.Organotin compounds and cancer chemotherapy.Coord Chem Rev,1989,95(1):109~123
收稿日期:1999-08-15, http://www.100md.com