+Gz重复暴露对大鼠脑组织热休克蛋白70基因表达的影响
作者:蔡庆 刘红巾 陈友纯 纪桂英 陈同欣
单位:蔡庆.空军总医院分子生物学研究中心,北京 100036
关键词:正加速度;基因表达;热休克蛋白70;大鼠
航天医学与医学工程990501Expression of HSP70 Gene in Rat Brain after Exposures
to Repeated +Gz
CAI Qing1, LIU Hong-jin2, CHEN You-chun1, JI Gui-ying2, CHEN Tong-xin2
(1.Molecular Biology Research Center, General Hospital of The Air Force, Beijing 100036;2.Clinical Aeromedical Center, General Hospital of The Air Force, Beijing 100036)
, 百拇医药
Abstract:Objective To study the changes of mRNA expression of heat shock protein 70 (HSP70) in the rat brain exposed to repeated +Gz. Method The mRNA expression levels of HSP70 in rat brain were measured by semi-quantitative reverse transcription polymerase chain reaction (RT-PCR). Result The HSP70 mRNA expression levels in rat brains taken 30 min and 6 h after repeated +Gz exposures were significantly higher than those in control group, while the difference between the levels of control group and those of experimental rat brains taken 24 h after +Gz exposure was not significant. Conclusion It is suggested that HSP70 mRNA expression in rat brain can be induced by repeated +Gz exposures and the increased HSP70 mRNA expression may play an important role in self-protection against brain damage induced by+Gz exposures.
, 百拇医药
Key words:positive acceleration;gene expression;heat shock protein 70;rats
摘要:目的 探讨+Gz重复暴露后大鼠脑组织热休克蛋白70(HSP70)基因表达的变化及其在+Gz所致脑损伤中的作用。方法 用半定量反转录聚合酶链反应(RT-PCR)检测方法检测+Gz重复暴露大鼠脑组织HSP70 mRNA表达水平。结果 +Gz重复暴露后30 min、6 h大鼠脑组织HSP70 mRNA均有升高,分别是对照组的1.7倍和2.6倍,24 h恢复正常。结论 +Gz重复暴露可诱导大鼠脑组织HSP70 mRNA的表达, 可能与+Gz所致脑损害后神经细胞的自身保护有关。
中图分类号:R852.21 文献标识码:A 文章编号:1002-0837(1999)05-0313-05
Modern high performance aircrafts are capable of producing accelerations as high as +9Gz and sustaining for 15 s to 45 s that may exceed human physiologic tolerance. +Gz induced loss of consciousness (G-LOS) is not only the major factor limiting man's performance, but also the main threat for flying safety. It was found that repeated +Gz exposures could bring about effects on brain tissue similar to “ischemia and reperfusion or hypoxia and reoxygenation"[1],and induced brain damage[2]. An increase in the mRNA level of heat shock protein 70 (HSP70), a major inducible stress protein, has been detected in cerebral ischemia[3~6]. Changes in HSP70 gene expression might modulate neuronal response to injury and prevent post-ischemic changes that could lead to cell death[5,6]. In this study, the mRNA expression level of HSP70 in the rat brain after exposure to +Gz was measured by semi-quantitative reverse transcription polymerase chain reaction (RT-PCR), to explore the effect of HSP70 in brain damage induced by repeated +Gz exposures.
, http://www.100md.com
Methods
Animal preparation Twenty-four male Wistar rats, weighing 180 g to 220 g, were randomly divided into four groups (each n=6). Group A (+1Gz group) served as control, and group B, C, D were repeatedly exposed to +10 Gz. All rats were awake during +Gz exposures on animal centrifuge (radius of 2 m). The rats were restrained in a special frame 5 cm in diameter, 17 cm in length. The frames were clamped to the animal centrifuge arm such that the head of the rats directed toward the center shaft of the centrifuge for +Gz orientation. Rats of experimental groups were repeatedly exposed to +10 Gz, 3 time each for 3 min with 30 min intervals in between. The rate of +Gz onset was about 1G/s. Rats of control group underwent the similar process as described above, but exposed to +1Gz. Rats of experimental groups were decapitated 30 min (group B), 6 h (group C), 24 h (group D) after the last centrifuge run, respectively. Brains were immediately removed and cut into small pieces for RNA isolation. Tissue procurement of control group was the same as that of experimental groups.
, 百拇医药
RNA isolation Total RNA from the fresh tissue samples was extracted according to the protocol of Chomczinski and Sacchi[7]. The amount of RNA present was determined by UV absorption. The optical density (OD) ratio of OD260nm:OD280nm was 1.8 to 2.0 in all cases. RNA samples were stored at -80℃ till further processing in RT-PCR.
Reverse transcription Two microgram of total RNA was used for reverse transcription into complementary DNA (cDNA). RNA was denatured for 3 min at 65℃ and incubated for 90 min at 37℃ in a final volume of 20 μl in the presence of 0.5 mM dNTP,10 pmol random primers, 200 units M-MLV reverse transcriptase (Promega) in 50 mM Tris-HCl, pH 8.3, 75 mM KCl, 3 mM MgCl2 and 10 mM dithiothreitol. The mixture was then heated to 70℃for 10 min.
, http://www.100md.com
Polymerase chain reaction 5 μl of each cDNA sample was amplified in a total volume of 30 μl containing PCR buffer (10 mM Tris-HCl, pH 8.3, 50 mM KCl, and 1.5 mM MgCl2), 200 μM of each dNTP, 0.5 μM of each oligonucleotide primers and 2 units of Taq DNA polymerase (Promega). In order to quantitate the PCR products comparatively and confirm the integrity of the RNAs,we coamplified a housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in a companion tube. The HSP70 and GAPDH oligonucleotide primers were synthesized basing on the published gene sequence[8,9]. PCR was carried out in a DNA thermal cycler (Perkin Elmer Cetus 9600) with 32 cycles as follows: 94℃for 1 min (denaturing, 3 min in first cycle) , 58℃ for 1 min (annealing) and 72℃ for 1.5 min(extension). In all experiments, a negative H2O control and known amounts of plasmids containing the respective gene sequences were amplified to check the efficiency of the PCR.
, http://www.100md.com
Quantitative analysis of PCR products After amplification, 15 μl of each PCR product was electrophoresed on 2% agarose gel containing ethidium bromide (0.5 μg/ml) in 40 mM Tris-acetate, pH8.5, 2 mM EDTA(TAE buffer). PCR Marker (Sino-American Biotechnology Company, China) was used as a molecular size standard. The gel was photographed under UV light at the same exposure and developing time. The bands on the photonegative film were scanned by densitometry for quantification. The PCR product for HSP70 or GAPDH amplified from the same cDNA was electrophoresed in the same gel and the ratio of HSP70/GAPDH was determined to eliminate gel-to-gel or film-to-film variance.
, 百拇医药
Results
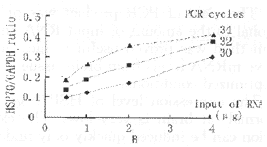
Optimization of PCR conditions The amplified product lengths for HSP70 and GAPDH obanited by RT-PCR were 419bp and 515bp fragments, respectively. To develop optimal conditions for RT-PCR, cDNAs were first synthesized from 4, 2, 1, and 0.5 μg of total RNAs from control rat brain. 5 μl of each cDNA was amplified at 30, 32 and 34 cycles. A 15 μl aliquot of each PCR cycle was electrophoresed on 2% agarose gel and analyzed quantitatively. PCR products for HSP70 were well correlated with the amount of RNA used and the number of PCR cycles (Fig. 1A). PCR products for GAPDH showed a slight increase according to the amount of RNA and PCR cycles. Figure 1B demonstrates the relationship between the amount of RNA and the relative HSP70 mRNA expression (the ratio of HSP70/GAPDH). At 34 cycles the ra-tio showed a plateau at 2 to 4 μg of RNA. At 30 cycles the ratio was linear but the sensitivity was so low that variance of the ratio between 0.5 and 2 μg RNA was small. At 32 cycles there was a clear increase in the ratio. For this study we used 2 μg of total RNA for cDNA synthesis and 32 cycles for PCR amplification.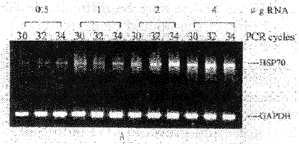
, 百拇医药
Fig.1 Optimization of PCR condition
A:electrophoresis of HSP70 RT-PCR product yield with the various amounts of RNA and various numbers of PCR cycles.
B:relative level of HSP70 mRNA (HSP70/GAPDH ratio) at various amounts of RNA and various numbers of PCR cycles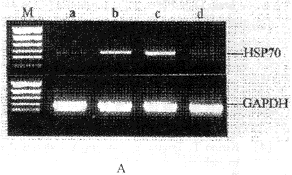

Fig.2 Changes of HSP70 mRNA levels in rat brain after repeated +10 Gz/3 min exposures(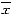 ±s,each group n=6)
±s,each group n=6)
, http://www.100md.com
a:control;b:30 min after +Gz exposure;c:6 h after +Gz exposure;d:24 h after +Gz exposure;M:PCR Marker.
A:electrophoresis of HSP70 RT-PCR product;B:changes of HSP70 mRNA levels (HSP70/GAPDH ratio)
*P<0.01,**P>0.05,as compared with control group
Altered expression of HSP70 in rat brain after exposure to repeated +Gz Figure 2A demonstrates the PCR product of group A (control), group B (30 min after repeated +Gz exposures), group C (6 h after repeated +Gz exposures) and group D (24 h after repeated +Gz exposures). The GAPDH bands of groups B, C and D shows approximately the same strength as that of the control group. Both group B and C showed an increased amount of the HSP70 band as compared to control group, but the HSP70 band of group D showed approximately the same strength as that of the control group.
, 百拇医药
Figure 2B demonstrates the changes in the relative levels of HSP70 mRNA expression of rat brains after repeated +Gz exposures. The HSP70 mRNA expression levels in the brains of group B and C were significantly increased (P<0.01) and were 1.7-fold, 2.6-fold as compared to that of control group, respectively. But the HSP70 mRNA levels in rat brains of group D were approximately the same as that of the control group (P>0.05).
Discussion
, http://www.100md.com
The quantitative methods for mRNA determination include Northern blot analysis, nuclease protection analysis, RT-PCR and so on. The quantitative RT-PCR has been popular to detect changes of mRNA levels in different tissues[10~14].With this technique, we can accurately determine the amounts of low abundance specific mRNAs in very limited amount of sample. In this study, semi-quantitative RT-PCR method for measuring HSP70 mRNA expression level was developed by optimizing the conditions for the amount of total RNA and the number of PCR amplification cycles. The yield of PCR product was proportional to the amount of input RNA and this method was proved useful to measure relative mRNA levels from brain tissue under optimized conditions.
, 百拇医药
The expression level of HSP70 gene in normal rat brain is very low. The expression can be induced quickly only under certain stimulations such as ischemia, hypoxia, high temperature or injury. More and more experimental proves have demonstrated that HSP70 mRNA is a sign of cell stress reaction, and the HSP70 protein may play an important role in protecting brain cells, strengthening the tolerence of brain cell to ischemia, hypoxia, or high temperature and resisting cell apoptosis[3~6]. Kirino[4] et al proved that the tolerance of the CA1 subfield neurons of the hippocampus in sabulosus mouse to ischemia can be enhanced by increased HSP70 gene expression in neurons. Lu[5] et al studied and showed that cell apoptosis is one of the molecular mechanisms of ischemic brain injury, and the HSP70 gene expression induced by ischemia mainly happens on the CA1 subfield neurons of the hippocampus suffering from stress injury and cell apoptosis, indicating that HSP70 expression is one of the self-protection functions of the neurons. Mailhos[6] et al also demonstrated that the HSP70 has the effect of protecting neurons and preventing the neurons from apoptosis.In this study, we measured the changes of mRNA expression of HSP70 in the brain of rat exposed to repeated +Gz. The results showed that the HSP70 mRNA expression levels in rat brains taken 30 min and 6 h after repeated +Gz exposures were significantly higher than those in control group, but the differences between the levels of control group and those of experimental rat brain taken 24 h after +Gz exposure were not significant. It suggests that HSP70 mRNA expression in rat brain can be induced by repeated +Gz exposures, and that the increased HSP70 mRNA expression may play an important role in self-protection against brain damage induced by+Gz exposures. But the mRNA expression levels of HSP70 returned to normal 24 h after +10Gz exposure, indicating that the period of the protection is limilted and it may be difficult for HSP70 to protect the injured neurons fully or for a considerablly long time.
, http://www.100md.com
This study demonstrated that HSP70 mRNA expression in rat brain can be induced by repeated +Gz exposure. The results provided the foundation for us to re-cognize and prevent +Gz induce brain injury at gene level.
References
[1]Glaster DH,Jobsis-Vander Vliet FF.A near-infrared spectrophotometric method for studying brain O2 sufficiency in man during +Gz acceleration[J].Aviat Space Environ Med,1988,59(3):199~207
, 百拇医药
[2]Shahed AR,Barber JA,Werchan PM.Multiple +Gz exposures cause brain edema in rats[J].Aviat Space Environ Med,1994,65(6):522~526
[3]Abe K,Tanzi RE,Kogure K.Induction of HSP70 mRNA after transient ischemia in gerbil brain[J].Neurosci Lett,1991,125(2):166~168
[4]Kirino T,Tsujita Y,Tamura A.Induced tolerance to ischemia in gerbil hippocampal neurons[J].J Cereb Blood Flow Metab 1991,11(2):299~307
, http://www.100md.com [5]Lu Dunyue,Jang Dehua.The gene expression of heat shock protein70 and cell apoptosis in rat hippocampal after global ischemic-reperfusion[J].Natl Med J China 1997,77(6):451~452
[6]Mailhos C,Howard MK,Latchman DS.Heat shock protein protects neuronal cells from programmed cell death by apoptosis[J].Neuroscience 1993,55(4):621~625
[7]Chomezynski P,Sacchi N.Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction[J].Anal Bioch,1987,162(1):156~159
, http://www.100md.com
[8]Longo FM,Wang S,Narasimhan P et al.cDNA cloning and expression of stress-inducible rat hsp70 in normal and injured rat brain[J].J Neurosci Res 1993,36(2):325~335
[9]Kaneto H,Morrissey J,Klahr S.Increased expression of TGF-β1 mRNA in the obstructed kidney of rats with unilateral ureteral ligation[J].Kidney,1993,44(2):313~321
[10]Caiqing,Liu Hongjin,Chen Youchen.Study of the expression of c-fos and c-jun gene in the brain of rat exposured to repeated +Gz by RT-PCR[J].Chin J Aerospeace Med,1998,9(2):77
, http://www.100md.com
[11]Kaneto H,Morrissey J,Klahr S.Increased expression of TGF-β1 mRNA in the obstructed kidney of rats with unilateral ureteral ligation[J].Kidney Int,1993,44(2):313~321
[12]Feldman AM,Ray PE,Silan CM et al.Selective gene expression in failing human heart: Quantification of steady-state levels of messenger RNA in endomyocardial biopsies using the polymerase chain reaction[J]. Circulation,1991,83(6):1866~1872
[13]Rocco MV,Neilson EG,Hoyer JR et al.Attenuated expression of epithelial cell adhesion molecules in murine polycystic kidney disease[J].Am J Physiol 1992,262(3):F679~686
[14]Ungerer M,Bohm M,Slce JS et al.Altered expression of β-Adrenergic receptors in the failing human heart[J].Circulation 1993,87(2):454~463
Recieved date:1998-11-10, 百拇医药
单位:蔡庆.空军总医院分子生物学研究中心,北京 100036
关键词:正加速度;基因表达;热休克蛋白70;大鼠
航天医学与医学工程990501Expression of HSP70 Gene in Rat Brain after Exposures
to Repeated +Gz
CAI Qing1, LIU Hong-jin2, CHEN You-chun1, JI Gui-ying2, CHEN Tong-xin2
(1.Molecular Biology Research Center, General Hospital of The Air Force, Beijing 100036;2.Clinical Aeromedical Center, General Hospital of The Air Force, Beijing 100036)
, 百拇医药
Abstract:Objective To study the changes of mRNA expression of heat shock protein 70 (HSP70) in the rat brain exposed to repeated +Gz. Method The mRNA expression levels of HSP70 in rat brain were measured by semi-quantitative reverse transcription polymerase chain reaction (RT-PCR). Result The HSP70 mRNA expression levels in rat brains taken 30 min and 6 h after repeated +Gz exposures were significantly higher than those in control group, while the difference between the levels of control group and those of experimental rat brains taken 24 h after +Gz exposure was not significant. Conclusion It is suggested that HSP70 mRNA expression in rat brain can be induced by repeated +Gz exposures and the increased HSP70 mRNA expression may play an important role in self-protection against brain damage induced by+Gz exposures.
, 百拇医药
Key words:positive acceleration;gene expression;heat shock protein 70;rats
摘要:目的 探讨+Gz重复暴露后大鼠脑组织热休克蛋白70(HSP70)基因表达的变化及其在+Gz所致脑损伤中的作用。方法 用半定量反转录聚合酶链反应(RT-PCR)检测方法检测+Gz重复暴露大鼠脑组织HSP70 mRNA表达水平。结果 +Gz重复暴露后30 min、6 h大鼠脑组织HSP70 mRNA均有升高,分别是对照组的1.7倍和2.6倍,24 h恢复正常。结论 +Gz重复暴露可诱导大鼠脑组织HSP70 mRNA的表达, 可能与+Gz所致脑损害后神经细胞的自身保护有关。
中图分类号:R852.21 文献标识码:A 文章编号:1002-0837(1999)05-0313-05
Modern high performance aircrafts are capable of producing accelerations as high as +9Gz and sustaining for 15 s to 45 s that may exceed human physiologic tolerance. +Gz induced loss of consciousness (G-LOS) is not only the major factor limiting man's performance, but also the main threat for flying safety. It was found that repeated +Gz exposures could bring about effects on brain tissue similar to “ischemia and reperfusion or hypoxia and reoxygenation"[1],and induced brain damage[2]. An increase in the mRNA level of heat shock protein 70 (HSP70), a major inducible stress protein, has been detected in cerebral ischemia[3~6]. Changes in HSP70 gene expression might modulate neuronal response to injury and prevent post-ischemic changes that could lead to cell death[5,6]. In this study, the mRNA expression level of HSP70 in the rat brain after exposure to +Gz was measured by semi-quantitative reverse transcription polymerase chain reaction (RT-PCR), to explore the effect of HSP70 in brain damage induced by repeated +Gz exposures.
, http://www.100md.com
Methods
Animal preparation Twenty-four male Wistar rats, weighing 180 g to 220 g, were randomly divided into four groups (each n=6). Group A (+1Gz group) served as control, and group B, C, D were repeatedly exposed to +10 Gz. All rats were awake during +Gz exposures on animal centrifuge (radius of 2 m). The rats were restrained in a special frame 5 cm in diameter, 17 cm in length. The frames were clamped to the animal centrifuge arm such that the head of the rats directed toward the center shaft of the centrifuge for +Gz orientation. Rats of experimental groups were repeatedly exposed to +10 Gz, 3 time each for 3 min with 30 min intervals in between. The rate of +Gz onset was about 1G/s. Rats of control group underwent the similar process as described above, but exposed to +1Gz. Rats of experimental groups were decapitated 30 min (group B), 6 h (group C), 24 h (group D) after the last centrifuge run, respectively. Brains were immediately removed and cut into small pieces for RNA isolation. Tissue procurement of control group was the same as that of experimental groups.
, 百拇医药
RNA isolation Total RNA from the fresh tissue samples was extracted according to the protocol of Chomczinski and Sacchi[7]. The amount of RNA present was determined by UV absorption. The optical density (OD) ratio of OD260nm:OD280nm was 1.8 to 2.0 in all cases. RNA samples were stored at -80℃ till further processing in RT-PCR.
Reverse transcription Two microgram of total RNA was used for reverse transcription into complementary DNA (cDNA). RNA was denatured for 3 min at 65℃ and incubated for 90 min at 37℃ in a final volume of 20 μl in the presence of 0.5 mM dNTP,10 pmol random primers, 200 units M-MLV reverse transcriptase (Promega) in 50 mM Tris-HCl, pH 8.3, 75 mM KCl, 3 mM MgCl2 and 10 mM dithiothreitol. The mixture was then heated to 70℃for 10 min.
, http://www.100md.com
Polymerase chain reaction 5 μl of each cDNA sample was amplified in a total volume of 30 μl containing PCR buffer (10 mM Tris-HCl, pH 8.3, 50 mM KCl, and 1.5 mM MgCl2), 200 μM of each dNTP, 0.5 μM of each oligonucleotide primers and 2 units of Taq DNA polymerase (Promega). In order to quantitate the PCR products comparatively and confirm the integrity of the RNAs,we coamplified a housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in a companion tube. The HSP70 and GAPDH oligonucleotide primers were synthesized basing on the published gene sequence[8,9]. PCR was carried out in a DNA thermal cycler (Perkin Elmer Cetus 9600) with 32 cycles as follows: 94℃for 1 min (denaturing, 3 min in first cycle) , 58℃ for 1 min (annealing) and 72℃ for 1.5 min(extension). In all experiments, a negative H2O control and known amounts of plasmids containing the respective gene sequences were amplified to check the efficiency of the PCR.
, http://www.100md.com
Quantitative analysis of PCR products After amplification, 15 μl of each PCR product was electrophoresed on 2% agarose gel containing ethidium bromide (0.5 μg/ml) in 40 mM Tris-acetate, pH8.5, 2 mM EDTA(TAE buffer). PCR Marker (Sino-American Biotechnology Company, China) was used as a molecular size standard. The gel was photographed under UV light at the same exposure and developing time. The bands on the photonegative film were scanned by densitometry for quantification. The PCR product for HSP70 or GAPDH amplified from the same cDNA was electrophoresed in the same gel and the ratio of HSP70/GAPDH was determined to eliminate gel-to-gel or film-to-film variance.
, 百拇医药
Results
Optimization of PCR conditions The amplified product lengths for HSP70 and GAPDH obanited by RT-PCR were 419bp and 515bp fragments, respectively. To develop optimal conditions for RT-PCR, cDNAs were first synthesized from 4, 2, 1, and 0.5 μg of total RNAs from control rat brain. 5 μl of each cDNA was amplified at 30, 32 and 34 cycles. A 15 μl aliquot of each PCR cycle was electrophoresed on 2% agarose gel and analyzed quantitatively. PCR products for HSP70 were well correlated with the amount of RNA used and the number of PCR cycles (Fig. 1A). PCR products for GAPDH showed a slight increase according to the amount of RNA and PCR cycles. Figure 1B demonstrates the relationship between the amount of RNA and the relative HSP70 mRNA expression (the ratio of HSP70/GAPDH). At 34 cycles the ra-tio showed a plateau at 2 to 4 μg of RNA. At 30 cycles the ratio was linear but the sensitivity was so low that variance of the ratio between 0.5 and 2 μg RNA was small. At 32 cycles there was a clear increase in the ratio. For this study we used 2 μg of total RNA for cDNA synthesis and 32 cycles for PCR amplification.


, 百拇医药
Fig.1 Optimization of PCR condition
A:electrophoresis of HSP70 RT-PCR product yield with the various amounts of RNA and various numbers of PCR cycles.
B:relative level of HSP70 mRNA (HSP70/GAPDH ratio) at various amounts of RNA and various numbers of PCR cycles


Fig.2 Changes of HSP70 mRNA levels in rat brain after repeated +10 Gz/3 min exposures(
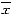 ±s,each group n=6)
±s,each group n=6), http://www.100md.com
a:control;b:30 min after +Gz exposure;c:6 h after +Gz exposure;d:24 h after +Gz exposure;M:PCR Marker.
A:electrophoresis of HSP70 RT-PCR product;B:changes of HSP70 mRNA levels (HSP70/GAPDH ratio)
*P<0.01,**P>0.05,as compared with control group
Altered expression of HSP70 in rat brain after exposure to repeated +Gz Figure 2A demonstrates the PCR product of group A (control), group B (30 min after repeated +Gz exposures), group C (6 h after repeated +Gz exposures) and group D (24 h after repeated +Gz exposures). The GAPDH bands of groups B, C and D shows approximately the same strength as that of the control group. Both group B and C showed an increased amount of the HSP70 band as compared to control group, but the HSP70 band of group D showed approximately the same strength as that of the control group.
, 百拇医药
Figure 2B demonstrates the changes in the relative levels of HSP70 mRNA expression of rat brains after repeated +Gz exposures. The HSP70 mRNA expression levels in the brains of group B and C were significantly increased (P<0.01) and were 1.7-fold, 2.6-fold as compared to that of control group, respectively. But the HSP70 mRNA levels in rat brains of group D were approximately the same as that of the control group (P>0.05).
Discussion
, http://www.100md.com
The quantitative methods for mRNA determination include Northern blot analysis, nuclease protection analysis, RT-PCR and so on. The quantitative RT-PCR has been popular to detect changes of mRNA levels in different tissues[10~14].With this technique, we can accurately determine the amounts of low abundance specific mRNAs in very limited amount of sample. In this study, semi-quantitative RT-PCR method for measuring HSP70 mRNA expression level was developed by optimizing the conditions for the amount of total RNA and the number of PCR amplification cycles. The yield of PCR product was proportional to the amount of input RNA and this method was proved useful to measure relative mRNA levels from brain tissue under optimized conditions.
, 百拇医药
The expression level of HSP70 gene in normal rat brain is very low. The expression can be induced quickly only under certain stimulations such as ischemia, hypoxia, high temperature or injury. More and more experimental proves have demonstrated that HSP70 mRNA is a sign of cell stress reaction, and the HSP70 protein may play an important role in protecting brain cells, strengthening the tolerence of brain cell to ischemia, hypoxia, or high temperature and resisting cell apoptosis[3~6]. Kirino[4] et al proved that the tolerance of the CA1 subfield neurons of the hippocampus in sabulosus mouse to ischemia can be enhanced by increased HSP70 gene expression in neurons. Lu[5] et al studied and showed that cell apoptosis is one of the molecular mechanisms of ischemic brain injury, and the HSP70 gene expression induced by ischemia mainly happens on the CA1 subfield neurons of the hippocampus suffering from stress injury and cell apoptosis, indicating that HSP70 expression is one of the self-protection functions of the neurons. Mailhos[6] et al also demonstrated that the HSP70 has the effect of protecting neurons and preventing the neurons from apoptosis.In this study, we measured the changes of mRNA expression of HSP70 in the brain of rat exposed to repeated +Gz. The results showed that the HSP70 mRNA expression levels in rat brains taken 30 min and 6 h after repeated +Gz exposures were significantly higher than those in control group, but the differences between the levels of control group and those of experimental rat brain taken 24 h after +Gz exposure were not significant. It suggests that HSP70 mRNA expression in rat brain can be induced by repeated +Gz exposures, and that the increased HSP70 mRNA expression may play an important role in self-protection against brain damage induced by+Gz exposures. But the mRNA expression levels of HSP70 returned to normal 24 h after +10Gz exposure, indicating that the period of the protection is limilted and it may be difficult for HSP70 to protect the injured neurons fully or for a considerablly long time.
, http://www.100md.com
This study demonstrated that HSP70 mRNA expression in rat brain can be induced by repeated +Gz exposure. The results provided the foundation for us to re-cognize and prevent +Gz induce brain injury at gene level.
References
[1]Glaster DH,Jobsis-Vander Vliet FF.A near-infrared spectrophotometric method for studying brain O2 sufficiency in man during +Gz acceleration[J].Aviat Space Environ Med,1988,59(3):199~207
, 百拇医药
[2]Shahed AR,Barber JA,Werchan PM.Multiple +Gz exposures cause brain edema in rats[J].Aviat Space Environ Med,1994,65(6):522~526
[3]Abe K,Tanzi RE,Kogure K.Induction of HSP70 mRNA after transient ischemia in gerbil brain[J].Neurosci Lett,1991,125(2):166~168
[4]Kirino T,Tsujita Y,Tamura A.Induced tolerance to ischemia in gerbil hippocampal neurons[J].J Cereb Blood Flow Metab 1991,11(2):299~307
, http://www.100md.com [5]Lu Dunyue,Jang Dehua.The gene expression of heat shock protein70 and cell apoptosis in rat hippocampal after global ischemic-reperfusion[J].Natl Med J China 1997,77(6):451~452
[6]Mailhos C,Howard MK,Latchman DS.Heat shock protein protects neuronal cells from programmed cell death by apoptosis[J].Neuroscience 1993,55(4):621~625
[7]Chomezynski P,Sacchi N.Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction[J].Anal Bioch,1987,162(1):156~159
, http://www.100md.com
[8]Longo FM,Wang S,Narasimhan P et al.cDNA cloning and expression of stress-inducible rat hsp70 in normal and injured rat brain[J].J Neurosci Res 1993,36(2):325~335
[9]Kaneto H,Morrissey J,Klahr S.Increased expression of TGF-β1 mRNA in the obstructed kidney of rats with unilateral ureteral ligation[J].Kidney,1993,44(2):313~321
[10]Caiqing,Liu Hongjin,Chen Youchen.Study of the expression of c-fos and c-jun gene in the brain of rat exposured to repeated +Gz by RT-PCR[J].Chin J Aerospeace Med,1998,9(2):77
, http://www.100md.com
[11]Kaneto H,Morrissey J,Klahr S.Increased expression of TGF-β1 mRNA in the obstructed kidney of rats with unilateral ureteral ligation[J].Kidney Int,1993,44(2):313~321
[12]Feldman AM,Ray PE,Silan CM et al.Selective gene expression in failing human heart: Quantification of steady-state levels of messenger RNA in endomyocardial biopsies using the polymerase chain reaction[J]. Circulation,1991,83(6):1866~1872
[13]Rocco MV,Neilson EG,Hoyer JR et al.Attenuated expression of epithelial cell adhesion molecules in murine polycystic kidney disease[J].Am J Physiol 1992,262(3):F679~686
[14]Ungerer M,Bohm M,Slce JS et al.Altered expression of β-Adrenergic receptors in the failing human heart[J].Circulation 1993,87(2):454~463
Recieved date:1998-11-10, 百拇医药