恒速静滴时5-FU血药浓度及毒性时辰变化的初步探讨
作者:李宇红,徐瑞华,廖海,黄慧强,崔景华,何友兼
单位:中山医科大学肿瘤防治中心内科(广州,510060)
关键词:时辰药理学;氟尿嘧啶/治疗应用;鼻咽肿瘤/治疗
癌症990620 【摘要】目的:探讨夜间、白天恒速静滴5-氟尿嘧啶(5-fluorouracil,5-FU)时,其稳态血药浓度及毒性的时辰变化,为制定适合我国患者的5-FU给药方案提供依据。方法:文应用高效液相色谱法测定5-FU血药浓度。18例晚期鼻咽癌患者前后两个疗程随机接受CF、5-FU白天及夜间化疗5天,5-FU1000mg.(m2.d)-1连续8小时恒速静滴,CF100mg在5-FU静滴第0、4、8小时,分别三次静推,卡铂300~350mg/m2,第6日静滴。对其中12例患者在每疗程5-FU静滴后第2、4、6、8小时取静脉血测定血药浓度。结果:恒速静滴5-FU其稳态血药浓度(Css)存在明显昼夜节律波动,高峰在1AM,夜间药时曲线下面积(AUC)高于白天。夜间静滴,本方案的主要剂量限制性毒性口腔粘膜炎,与5-FU高峰血药浓度、AUC相关,但在白天静滴,这种相关性不明显。5-FU白天、夜间恒速静滴,该方案毒副反应无差异。结论:5-FU毒性可能与其血药浓度及靶组织敏感性等因素有关,推测5-FU较合理给药方式应是24小时连续静滴,高峰滴速在3AM-5AM,因为这种给药方式避免了在高峰血药浓度及口腔粘膜细胞DNA合成活跃的时间给予较高浓度的5-FU。
, http://www.100md.com
中图分类号:R96;R979.1;R739.63 文献标识码:A 文章编号:1000-467X(1999)06-0694-03
A pilot study of 5-FU circadian pharmacokinetics in patients with
nasopharyngeal carcinoma
LI Yu-hong, XU Rui-hua, LIAO Hai, et al.
Department of Medical Oncology,Cancer Center,Sun Yat-sen University of Medical Sciences, Guangzhou 510060,P.R.China
【Abstract】Objective:While 5-FU was administered during day or night, its stead-steady plasma concentration (Css) and toxicity of the regimens were compared so as to find out more effective administration of 5FU .Methods:A tolal of 18 patients randomly received CF plus 5-FU during day or night. 5-FU 1000 mg.(m2.d)-1 was given by 8 hours continuous intravenously infusion (IV) for 5 days (d1~5),leucovorion 100 mg was administered after 5-FU infusion at 0,4,8 hour,together with carboplatin 300~350 mg/m2,was administred IV on d6.Using HPLC method, plasma concentrationg of 5-FU were detected in 12 patients at 2,4,6,8 hour after 5-FU infusion.Results:Pharmacokinetics data showed although 5-FU was continuously infused at a constant rate,the Css of this drug varied in a circadian rhythmic manner, with maximal values occuring at 1AM.Area under concentration curve (AUC) of 5-FU at night was significantly higher than that during day. While CF flus 5-FU were administered at night, close positive correlation between 5-FU pharmacokinetics data (maximum Css,AUC) and the risk of mucositis were found. But in the daytime,these relationship was insignificant.No difference in toxicity was observed while CF flus 5-FU were administered during day or night at constant rate.Conclusion:It is suggested that toxicity of 5-FU may be related to its plasma concentration and sensitivity of target tissue. The optimal administration of 5-FU should be continuous IV, with peak between 3AM to 5AM,because it is helpful to avoid administration of 5-FU during its maximum plasma concentration and active DNA synthesis of oral mucosa.
, 百拇医药
Key words:Chronopharmacology;Fluorouracil; Nasopharyngeal neoplasms
5-氟尿嘧啶(5-fluorouracil,5-FU)是常见的抗肿瘤药物之一,已有四十多年历史。近年来,国外有文献报道5-FU毒性及血药浓度变化具有时辰性,而国内则未见有关报道,我们监测白天及夜间恒速静滴给药时,5-FU在中国肿瘤病人体内稳态血药浓度变化情况,对5-FU时辰用药进行初步探讨,为制定适合我国肿瘤患者的5-FU给药方案提供依据。
1资料与方法
1.1病例选择及给药方法
经病理证实晚期(Ⅲ、Ⅳ期)初治或放疗、化疗后局部复发/或远处转移的鼻咽癌病例,以往化疗停止1月以上,主要器官功能正常。符合入选条件患者自身前后两个疗程随机接受CF、5-FU白天、夜间化疗。白天化疗方案:5-FU1000mg.(m2.d)-1,9AM至5PM静脉泵恒速滴注,第一天至第五天(d1~d5),醛氢叶酸(CF)100mg9AM、1PM、5PM3次静脉推注,d1~d5,卡铂300~350mg/m2,9AM静注,d6。夜间化疗方案:5-FU1000mg.(m2.d)-1,9PM至5AM静脉泵恒速滴注,d1~d5,CF100mg9PM、1AM、5AM3次静脉推注,d1~d5,卡铂同白天化疗。
, 百拇医药
1.2血药浓度测定
1.2.1试剂和仪器:标准品5-FU、内标5溴尿嘧啶(5Bu)为上海试剂二厂产品,5-FU注射液为南通制药总厂产品,高效液相色谱(HPLC)试验仪器由美国Varian公司生产。
1.2.2测定条件:色谱柱为美国ZoRBAXC18反相柱(4.6mm×250mm),流动相为H3PO4-Na2HPO4缓冲液+5%甲醇(pH3.2),流速1ml/min,压力1.52×104kPa~1.82×104kPa,紫外检测波长269nm,灵敏度0.05AUFS,记录纸速8mm/min,柱温16±1℃下操作。
1.2.3同一患者分别在第一、二疗程,在5-FU给药后第2,4,6,8小时在另一侧肘静脉取血3~5ml置肝素管中,离心取上层血清,置-20℃冰箱冷藏待测。
, 百拇医药
1.2.4数据处理:峰面积计算及峰型记录采用Varian公司提供的计算软件。静脉连续滴注,AUC采用梯形法计算。
1.3毒性观察
化疗前及化疗后每周1次检查患者外周血象,每个疗程前后复查肝肾功能,血电解质等生化指标及心电图,详细记录化疗后各系统不良反应,观察指标采用WHO毒性分级标准。
2结果
2.1白天及夜间静滴5-FU稳态血药浓度比较
2.1.1本法在5-FU血药浓度从0.05mg/L至100mg/L的范围有良好的线性关系。Y=0.2745X+5.2478,相关系数r=0.9995。回收率为79.2%±8%。日内变异系数(CV)=2.84%±5.95%,日间变异系数(CV)=2.13%±9.13%。
, http://www.100md.com
2.2.2对12例患者进行5-FU稳态血药浓度检测,结果不同个体5-FU平均血药浓度在同一时间点有较大数值标准差,用多个样本均数间每两个样本均数的比较(q检验),1AM时稳态血药浓度与其它时间点有统计学差异(P<0.05),其余各时间点无统计学差异(P>0.05)(表1)。12例患者AUC夜间高于白天,用配对t检验,两组有统计学差异(表2)。
表1 12例患者在各时间点的平均稳态血药浓度
时间点
平均稳态血药浓度(mg/L,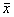 ±s)
±s)
11PM
15.2489±23.8288
1AM
, http://www.100md.com
174.1875±356.0119
3AM
48.9873±77.3169
5AM
11.6870±15.9389
11AM
8.3047±11.2331
1PM
8.5799±14.1855
3PM
5.360±2.9600
5PM
, 百拇医药
5.3407±3.1706
1AM时Css与其它各时间点有统计学差异(P<0.05),其余各时间点间无统计学差异。(q检验)
表2 12例患者夜间与白天静滴时药时曲线下面积(AUC)比较
患者号
夜间AUC(mg.h-1.L-1)
白天AUC(mg.h-1.L-1)
1
45.7217
51.5564
, http://www.100md.com
2
77.2499
48.049
3
42.9248
30.4111
4
46.1020
32.1551
5
1547.9224
229.897
6
, http://www.100md.com
947.9070
26.2448
7
329.688
25.9713
8
36.5889
53.0001
9
426.5847
16.7796
10
7.1336
, 百拇医药
31.6295
11
46.2306
40.9852
12
42.2304
7.2958
夜间AUC高于白天,P=0.0471。(配对t检验)
2.2 5-FU血药浓度及AUC与毒性及疗效关系
夜间静滴,本联合化疗方案的主要剂量限制性毒性口腔粘膜炎与5-FU高峰血药浓度相关系数r=0.6136,用Spearman等级相关检验P=0.0338(P<0.05)。口腔粘膜炎与AUC相关系数r=0.6145,用Spearman等级相关检验P=0.0335(P<0.05)(表3)。白天静滴,口腔粘膜炎与5-FU高峰血药浓度相关系数r=-0.2559,Spearman等级相关检验P=0.4221(P>0.05),无相关性。口腔粘膜炎与AUC相关系数r=-0.2327,Spearman等级相关检验P=0.4667(P>0.05),无相关性(表4)。5-FU夜间、白天高峰血药浓度及AUC与疗效无相关性。(表3、4)
, 百拇医药
表3 夜间静滴时,Css、AUC与口腔粘膜炎及疗效的关系
患者号
口腔粘膜炎
疗效
高峰Css(mg/L)
AUC(mg.h-1.L-1)
1
Ⅲ
PR
7.7582
45.7217
, 百拇医药
2
Ⅲ
PR
13.7976
77.2499
3
Ⅱ
PR
8.2346
42.9248
4
Ⅱ
CR
, http://www.100md.com
8.8061
46.1020
5
Ⅲ
MR
751.3478
1547.9224
6
Ⅳ
MR
224.679
947.9070
7
, 百拇医药
Ⅳ
PR
1070.0931
329.688
8
0
PR
7.4652
36.5889
9
Ⅲ
PD
149.3718
, 百拇医药
426.5847
10
0
PR
1.3909
7.1336
11
0
PR
8.7011
46.2306
12
0
, 百拇医药
PR
8.9019
42.2304
夜间高峰Css与口腔粘膜炎相关系数r=0.6136,P=0.0338。夜间AUC与口腔粘膜炎相关系数r=0.6136,P=0.0338。夜间AUC及高峰Css与疗效无相关性
表4 白天静滴时,Css、AUC与口腔粘膜炎及疗效的关系
患者号
口腔粘膜炎
疗效
高峰Css(mg/L)
AUC(mg.h-1.L-1)
, 百拇医药
1
Ⅲ
PR
8.0620
51.5564
2
Ⅱ
PR
8.5295
48.049
3
0
PR
, http://www.100md.com
9.0395
30.4111
4
Ⅱ
CR
4.8979
32.1551
5
Ⅱ
MR
53.6609
229.897
6
, http://www.100md.com
Ⅳ
MR
5.2386
26.2488
7
Ⅳ
PR
5.3204
25.9713
8
0
PR
9.0059
, 百拇医药
53.0001
9
Ⅱ
PD
3.4463
16.7796
10
0
PR
7.3018
31.6259
11
Ⅰ
, 百拇医药
PR
6.3563
40.9852
12
0
PR
2.9957
7.2958
白天高峰Css与口腔粘膜炎相关系数r=-0.2559,P=0.4221。白天AUC与口腔粘膜炎相关系数r=0.2327,P=0.4667。白天AUC及高峰Css与疗效无相关性
2.3白天与夜间5-FU化疗毒性反应比较
18例患者进入临床试验,该方案主要不良反应包括口腔粘膜炎,骨髓抑制,恶心呕吐,腹泻,脱发,静脉炎,色素沉着,急性皮炎,白天及夜间化疗毒副反应类型相同,发生率未见统计学差异(P>0.05)(表5)。
, 百拇医药
表5 5-FU CF夜间及白天给药的主要不良反应 例(%)
不良
反应
0
夜间(18疗程)不良反应级别
0
白天(18疗程)不良反应级别
P值*
Ⅰ
Ⅱ
Ⅲ
Ⅳ
, 百拇医药
Ⅰ
Ⅱ
Ⅲ
Ⅳ
口腔粘膜炎
6(33.3)
0
6(3.33)
3(16.7)
3(16.7)
5(27.8)
3(16.7)
6(33.3)
, http://www.100md.com
2(11.1)
2(11.1)
>0.05
WBC↓
8(44.4)
4(22.2)
5(27.8)
0
1(5.6)
9(50)
3(16.7)
4(22.2)
, 百拇医药 1(11.1)
1(5.6)
>0.05
HB↓
16(88.8)
1(5.6)
1(5.6)
0
0
16(88.8)
0
2(11.2)
0
, 百拇医药
0
>0.05
PLT↓
14(73.7)
2(11.1)
0
1(5.6)
1(5.6)
14(77.8)
0
3(16.7)
1(5.6)
0
, 百拇医药
>0.05
恶心
呕吐
9(50)
8(44.4)
2(11.1)
0
0
5(27.8)
11(61.1)
2(11.1)
0
0
, 百拇医药
>0.05
腹泻
14(77.8)
2(11.1)
2(11.1)
0
0
16(88.9)
0
0
0
0
>0.05
, 百拇医药
静脉炎
13(72.2)
5(27.8)
0
0
0
13(72.2)
5(27.8)
0
0
0
>0.05
脱发
, 百拇医药
5(27.8)
10(55.6)
2(11.1)
1(5.6)
0
5(27.8)
5(27.8)
7(38.9)
1(5.6)
0
>0.05
色素
沉着
, http://www.100md.com
11(61.1)
7(38.9)
0
0
0
11(61.1)
7(38.9)
0
0
0
>0.05
急性
皮炎
, 百拇医药
15(83.3)
3(17.7)
0
0
0
15(83.3)
3(17.7)
0
0
0
>0.05
*采用Ridit分析
3讨论
, 百拇医药
3.1 5-FU稳态血药浓度变化及与该方案毒性反应关系
静脉推注时,5-FU血浆半衰期为10~20分钟,当我们以125mg.(m2.h)-1恒速静滴5-FU2小时后,理论上血浆中5-FU应达稳态血药浓度。但在本实验中,我们对12名患者白天及夜间的稳态血药浓度进行检测,结果表明其并非维持在一定水平或在一定水平上下波动,而是呈明显的昼夜节律变化,最高峰大多在1AM。国外文献有类似报道〔1-3〕,国内则未见。其机理尚不清楚,被认为可能与5-FU重要分解限速酶二氢尿嘧啶脱氢酶(DPD)的昼夜节律变化有关〔4〕。
本试验中我们发现夜间静滴,5-FU血药浓度及AUC与本方案主要剂量限制性毒性口腔粘膜炎相关,白天静滴无相关性。故认为夜间静滴5-FU,其血药浓度、AUC高低可能是影响口腔粘膜的主要因素,而白天并非如此。骨髓、胃肠道粘膜上皮细胞合成活跃,是5-FU化疗产生毒性最常见组织,有文献报道胃肠道粘膜上皮细胞DNA合成有较明显昼夜节律,早晨比夜晚活跃〔5〕。患者白天口腔粘膜细胞DNA合成活跃,即使在5-FU血药浓度及AUC较低时,也可能造成对口腔粘膜损伤。故我们建议对于24小时恒速静滴患者,检测其24小时AUC值,特别是夜间血药浓度及AUC值,对于调整5-FU剂量,降低毒性意义更大。
, 百拇医药
3.25-FU合理给药方式的推测
本实验结果显示,5-FU夜间静滴时AUC高于白天,高峰血药浓度时间在1AM,而3AM、5AM时5-FU血药浓度与白天无统计学差异。故我们认为在3AM至5AM给予5-FU可获得较低毒副反应,因为这段时间给药既可避免5-FU在1AM时高峰血药浓度对口腔粘膜的毒性,又避免在口腔粘膜细胞,骨髓细胞DNA合成活跃,耐受性差的白天给予较高浓度5-FU。24小时连续静脉给予5-FU毒性低、疗效好,已成为国外普遍采用的给药方式,在晚期肠癌化疗中,随机临床试验已证实与静脉推注相比,能提高有效率〔6,7〕。故我们认为5-FU较合理给药方式应是24小时连续静滴,高峰滴速在3AM至5AM。这与国外文献对5-FU24小时连续静滴,高峰滴速在4AM时辰给药方式相似〔8〕。在本临床研究中,5-FU给药无法做到变速,虽然患者夜间口腔粘膜细胞DNA合成少,耐受性良好,但因有较高的5-FU高峰血药浓度及AUC,使夜间与白天毒副反应无明显差别。随着可携带的电脑调控程序静脉泵在国内的应用,我们将在肠癌、鼻咽癌、乳腺癌等肿瘤化疗中,对5-FU时辰给药方式与连续恒速静滴进行随机对照研究,进一步评价5-FU时辰给药的临床价值。
, http://www.100md.com
作者简介:何友兼 通讯作者
〔参考文献〕
〔1〕Harris BE,Song R, Soong ST, et al. Relationship between dihydropyrimidine dehydrogenase activity and plasma 5-fluorouracil levels with evidence for circadian variation of enzyme activity and plasma drug levels in cancer patients receiving 5-fluorouracil by protracted continuous infusion〔J〕.Cancer Res,1990,50:197~201.
〔2〕Metzger G,Massari C, Etienne MC, et al. Spontaneous or imposed circadian changes in plasma concentrations of 5-fluorouracil coadministered with folinic acid and oxaliplatin:relationship with mucosal toxicity in patients with cancer〔J〕.Clin Pharmacol Ther,1994, 56:190~201.
, 百拇医药
〔3〕Petit E,Milano G, Levi F,et al. Circadian rhythm-varying plasma concentration of 5-fluorouracil during a five day continuous infusion at a constant rate in cancer patients〔J〕.Cancer Res,1988,48:1676~1679.
〔4〕Hrushesky WJ, Bjarnason GA,Circadian cancer therapy〔J〕.J Clin Oncol,1993,11:1403~1417.
〔5〕Buchi KN,Moore JG, Hrushesky WJ,et al.Circadian rhythm of cellular proliferation in the human rectal mucosa 〔J〕.Gastroenterology,1991,101:410~415.
, http://www.100md.com
〔6〕Lokich JJ,Ahlgren JD,Gullo JJ,et al.A prospective randomized comparison of continous infusion fluorouracil with a conventional bolus schedule in metastatic colorectal carcinoma:a Mid-Atlantic Oncology Program Study 〔J〕.J Clin Oncol, 1989,7:425~432.
〔7〕Weinerman B,Shah A,Fields A,et al.A randomized trail of continous systemic infusion verus bolus therapy with 5-fluorouracil in metastatic measurable colorectal cancer〔J〕.Proc Am Soc Clin Oncol,1991,10:143.
〔8〕Levi F, Misset JL,Brienza S,et al.A chronopharmacologic phase II clinical trail with 5-fluorouracil,folinic acid and oxaliplatin using an ambulatory mutichannal programmable pump. High antitumor effetivaness against metastatic colorectal cancer 〔J〕. Cancer, 1992,69:893~900.
收稿日期:1998-08-11;修回日期:1998-10-21, http://www.100md.com
单位:中山医科大学肿瘤防治中心内科(广州,510060)
关键词:时辰药理学;氟尿嘧啶/治疗应用;鼻咽肿瘤/治疗
癌症990620 【摘要】目的:探讨夜间、白天恒速静滴5-氟尿嘧啶(5-fluorouracil,5-FU)时,其稳态血药浓度及毒性的时辰变化,为制定适合我国患者的5-FU给药方案提供依据。方法:文应用高效液相色谱法测定5-FU血药浓度。18例晚期鼻咽癌患者前后两个疗程随机接受CF、5-FU白天及夜间化疗5天,5-FU1000mg.(m2.d)-1连续8小时恒速静滴,CF100mg在5-FU静滴第0、4、8小时,分别三次静推,卡铂300~350mg/m2,第6日静滴。对其中12例患者在每疗程5-FU静滴后第2、4、6、8小时取静脉血测定血药浓度。结果:恒速静滴5-FU其稳态血药浓度(Css)存在明显昼夜节律波动,高峰在1AM,夜间药时曲线下面积(AUC)高于白天。夜间静滴,本方案的主要剂量限制性毒性口腔粘膜炎,与5-FU高峰血药浓度、AUC相关,但在白天静滴,这种相关性不明显。5-FU白天、夜间恒速静滴,该方案毒副反应无差异。结论:5-FU毒性可能与其血药浓度及靶组织敏感性等因素有关,推测5-FU较合理给药方式应是24小时连续静滴,高峰滴速在3AM-5AM,因为这种给药方式避免了在高峰血药浓度及口腔粘膜细胞DNA合成活跃的时间给予较高浓度的5-FU。
, http://www.100md.com
中图分类号:R96;R979.1;R739.63 文献标识码:A 文章编号:1000-467X(1999)06-0694-03
A pilot study of 5-FU circadian pharmacokinetics in patients with
nasopharyngeal carcinoma
LI Yu-hong, XU Rui-hua, LIAO Hai, et al.
Department of Medical Oncology,Cancer Center,Sun Yat-sen University of Medical Sciences, Guangzhou 510060,P.R.China
【Abstract】Objective:While 5-FU was administered during day or night, its stead-steady plasma concentration (Css) and toxicity of the regimens were compared so as to find out more effective administration of 5FU .Methods:A tolal of 18 patients randomly received CF plus 5-FU during day or night. 5-FU 1000 mg.(m2.d)-1 was given by 8 hours continuous intravenously infusion (IV) for 5 days (d1~5),leucovorion 100 mg was administered after 5-FU infusion at 0,4,8 hour,together with carboplatin 300~350 mg/m2,was administred IV on d6.Using HPLC method, plasma concentrationg of 5-FU were detected in 12 patients at 2,4,6,8 hour after 5-FU infusion.Results:Pharmacokinetics data showed although 5-FU was continuously infused at a constant rate,the Css of this drug varied in a circadian rhythmic manner, with maximal values occuring at 1AM.Area under concentration curve (AUC) of 5-FU at night was significantly higher than that during day. While CF flus 5-FU were administered at night, close positive correlation between 5-FU pharmacokinetics data (maximum Css,AUC) and the risk of mucositis were found. But in the daytime,these relationship was insignificant.No difference in toxicity was observed while CF flus 5-FU were administered during day or night at constant rate.Conclusion:It is suggested that toxicity of 5-FU may be related to its plasma concentration and sensitivity of target tissue. The optimal administration of 5-FU should be continuous IV, with peak between 3AM to 5AM,because it is helpful to avoid administration of 5-FU during its maximum plasma concentration and active DNA synthesis of oral mucosa.
, 百拇医药
Key words:Chronopharmacology;Fluorouracil; Nasopharyngeal neoplasms
5-氟尿嘧啶(5-fluorouracil,5-FU)是常见的抗肿瘤药物之一,已有四十多年历史。近年来,国外有文献报道5-FU毒性及血药浓度变化具有时辰性,而国内则未见有关报道,我们监测白天及夜间恒速静滴给药时,5-FU在中国肿瘤病人体内稳态血药浓度变化情况,对5-FU时辰用药进行初步探讨,为制定适合我国肿瘤患者的5-FU给药方案提供依据。
1资料与方法
1.1病例选择及给药方法
经病理证实晚期(Ⅲ、Ⅳ期)初治或放疗、化疗后局部复发/或远处转移的鼻咽癌病例,以往化疗停止1月以上,主要器官功能正常。符合入选条件患者自身前后两个疗程随机接受CF、5-FU白天、夜间化疗。白天化疗方案:5-FU1000mg.(m2.d)-1,9AM至5PM静脉泵恒速滴注,第一天至第五天(d1~d5),醛氢叶酸(CF)100mg9AM、1PM、5PM3次静脉推注,d1~d5,卡铂300~350mg/m2,9AM静注,d6。夜间化疗方案:5-FU1000mg.(m2.d)-1,9PM至5AM静脉泵恒速滴注,d1~d5,CF100mg9PM、1AM、5AM3次静脉推注,d1~d5,卡铂同白天化疗。
, 百拇医药
1.2血药浓度测定
1.2.1试剂和仪器:标准品5-FU、内标5溴尿嘧啶(5Bu)为上海试剂二厂产品,5-FU注射液为南通制药总厂产品,高效液相色谱(HPLC)试验仪器由美国Varian公司生产。
1.2.2测定条件:色谱柱为美国ZoRBAXC18反相柱(4.6mm×250mm),流动相为H3PO4-Na2HPO4缓冲液+5%甲醇(pH3.2),流速1ml/min,压力1.52×104kPa~1.82×104kPa,紫外检测波长269nm,灵敏度0.05AUFS,记录纸速8mm/min,柱温16±1℃下操作。
1.2.3同一患者分别在第一、二疗程,在5-FU给药后第2,4,6,8小时在另一侧肘静脉取血3~5ml置肝素管中,离心取上层血清,置-20℃冰箱冷藏待测。
, 百拇医药
1.2.4数据处理:峰面积计算及峰型记录采用Varian公司提供的计算软件。静脉连续滴注,AUC采用梯形法计算。
1.3毒性观察
化疗前及化疗后每周1次检查患者外周血象,每个疗程前后复查肝肾功能,血电解质等生化指标及心电图,详细记录化疗后各系统不良反应,观察指标采用WHO毒性分级标准。
2结果
2.1白天及夜间静滴5-FU稳态血药浓度比较
2.1.1本法在5-FU血药浓度从0.05mg/L至100mg/L的范围有良好的线性关系。Y=0.2745X+5.2478,相关系数r=0.9995。回收率为79.2%±8%。日内变异系数(CV)=2.84%±5.95%,日间变异系数(CV)=2.13%±9.13%。
, http://www.100md.com
2.2.2对12例患者进行5-FU稳态血药浓度检测,结果不同个体5-FU平均血药浓度在同一时间点有较大数值标准差,用多个样本均数间每两个样本均数的比较(q检验),1AM时稳态血药浓度与其它时间点有统计学差异(P<0.05),其余各时间点无统计学差异(P>0.05)(表1)。12例患者AUC夜间高于白天,用配对t检验,两组有统计学差异(表2)。
表1 12例患者在各时间点的平均稳态血药浓度
时间点
平均稳态血药浓度(mg/L,
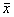 ±s)
±s)11PM
15.2489±23.8288
1AM
, http://www.100md.com
174.1875±356.0119
3AM
48.9873±77.3169
5AM
11.6870±15.9389
11AM
8.3047±11.2331
1PM
8.5799±14.1855
3PM
5.360±2.9600
5PM
, 百拇医药
5.3407±3.1706
1AM时Css与其它各时间点有统计学差异(P<0.05),其余各时间点间无统计学差异。(q检验)
表2 12例患者夜间与白天静滴时药时曲线下面积(AUC)比较
患者号
夜间AUC(mg.h-1.L-1)
白天AUC(mg.h-1.L-1)
1
45.7217
51.5564
, http://www.100md.com
2
77.2499
48.049
3
42.9248
30.4111
4
46.1020
32.1551
5
1547.9224
229.897
6
, http://www.100md.com
947.9070
26.2448
7
329.688
25.9713
8
36.5889
53.0001
9
426.5847
16.7796
10
7.1336
, 百拇医药
31.6295
11
46.2306
40.9852
12
42.2304
7.2958
夜间AUC高于白天,P=0.0471。(配对t检验)
2.2 5-FU血药浓度及AUC与毒性及疗效关系
夜间静滴,本联合化疗方案的主要剂量限制性毒性口腔粘膜炎与5-FU高峰血药浓度相关系数r=0.6136,用Spearman等级相关检验P=0.0338(P<0.05)。口腔粘膜炎与AUC相关系数r=0.6145,用Spearman等级相关检验P=0.0335(P<0.05)(表3)。白天静滴,口腔粘膜炎与5-FU高峰血药浓度相关系数r=-0.2559,Spearman等级相关检验P=0.4221(P>0.05),无相关性。口腔粘膜炎与AUC相关系数r=-0.2327,Spearman等级相关检验P=0.4667(P>0.05),无相关性(表4)。5-FU夜间、白天高峰血药浓度及AUC与疗效无相关性。(表3、4)
, 百拇医药
表3 夜间静滴时,Css、AUC与口腔粘膜炎及疗效的关系
患者号
口腔粘膜炎
疗效
高峰Css(mg/L)
AUC(mg.h-1.L-1)
1
Ⅲ
PR
7.7582
45.7217
, 百拇医药
2
Ⅲ
PR
13.7976
77.2499
3
Ⅱ
PR
8.2346
42.9248
4
Ⅱ
CR
, http://www.100md.com
8.8061
46.1020
5
Ⅲ
MR
751.3478
1547.9224
6
Ⅳ
MR
224.679
947.9070
7
, 百拇医药
Ⅳ
PR
1070.0931
329.688
8
0
PR
7.4652
36.5889
9
Ⅲ
PD
149.3718
, 百拇医药
426.5847
10
0
PR
1.3909
7.1336
11
0
PR
8.7011
46.2306
12
0
, 百拇医药
PR
8.9019
42.2304
夜间高峰Css与口腔粘膜炎相关系数r=0.6136,P=0.0338。夜间AUC与口腔粘膜炎相关系数r=0.6136,P=0.0338。夜间AUC及高峰Css与疗效无相关性
表4 白天静滴时,Css、AUC与口腔粘膜炎及疗效的关系
患者号
口腔粘膜炎
疗效
高峰Css(mg/L)
AUC(mg.h-1.L-1)
, 百拇医药
1
Ⅲ
PR
8.0620
51.5564
2
Ⅱ
PR
8.5295
48.049
3
0
PR
, http://www.100md.com
9.0395
30.4111
4
Ⅱ
CR
4.8979
32.1551
5
Ⅱ
MR
53.6609
229.897
6
, http://www.100md.com
Ⅳ
MR
5.2386
26.2488
7
Ⅳ
PR
5.3204
25.9713
8
0
PR
9.0059
, 百拇医药
53.0001
9
Ⅱ
PD
3.4463
16.7796
10
0
PR
7.3018
31.6259
11
Ⅰ
, 百拇医药
PR
6.3563
40.9852
12
0
PR
2.9957
7.2958
白天高峰Css与口腔粘膜炎相关系数r=-0.2559,P=0.4221。白天AUC与口腔粘膜炎相关系数r=0.2327,P=0.4667。白天AUC及高峰Css与疗效无相关性
2.3白天与夜间5-FU化疗毒性反应比较
18例患者进入临床试验,该方案主要不良反应包括口腔粘膜炎,骨髓抑制,恶心呕吐,腹泻,脱发,静脉炎,色素沉着,急性皮炎,白天及夜间化疗毒副反应类型相同,发生率未见统计学差异(P>0.05)(表5)。
, 百拇医药
表5 5-FU CF夜间及白天给药的主要不良反应 例(%)
不良
反应
0
夜间(18疗程)不良反应级别
0
白天(18疗程)不良反应级别
P值*
Ⅰ
Ⅱ
Ⅲ
Ⅳ
, 百拇医药
Ⅰ
Ⅱ
Ⅲ
Ⅳ
口腔粘膜炎
6(33.3)
0
6(3.33)
3(16.7)
3(16.7)
5(27.8)
3(16.7)
6(33.3)
, http://www.100md.com
2(11.1)
2(11.1)
>0.05
WBC↓
8(44.4)
4(22.2)
5(27.8)
0
1(5.6)
9(50)
3(16.7)
4(22.2)
, 百拇医药 1(11.1)
1(5.6)
>0.05
HB↓
16(88.8)
1(5.6)
1(5.6)
0
0
16(88.8)
0
2(11.2)
0
, 百拇医药
0
>0.05
PLT↓
14(73.7)
2(11.1)
0
1(5.6)
1(5.6)
14(77.8)
0
3(16.7)
1(5.6)
0
, 百拇医药
>0.05
恶心
呕吐
9(50)
8(44.4)
2(11.1)
0
0
5(27.8)
11(61.1)
2(11.1)
0
0
, 百拇医药
>0.05
腹泻
14(77.8)
2(11.1)
2(11.1)
0
0
16(88.9)
0
0
0
0
>0.05
, 百拇医药
静脉炎
13(72.2)
5(27.8)
0
0
0
13(72.2)
5(27.8)
0
0
0
>0.05
脱发
, 百拇医药
5(27.8)
10(55.6)
2(11.1)
1(5.6)
0
5(27.8)
5(27.8)
7(38.9)
1(5.6)
0
>0.05
色素
沉着
, http://www.100md.com
11(61.1)
7(38.9)
0
0
0
11(61.1)
7(38.9)
0
0
0
>0.05
急性
皮炎
, 百拇医药
15(83.3)
3(17.7)
0
0
0
15(83.3)
3(17.7)
0
0
0
>0.05
*采用Ridit分析
3讨论
, 百拇医药
3.1 5-FU稳态血药浓度变化及与该方案毒性反应关系
静脉推注时,5-FU血浆半衰期为10~20分钟,当我们以125mg.(m2.h)-1恒速静滴5-FU2小时后,理论上血浆中5-FU应达稳态血药浓度。但在本实验中,我们对12名患者白天及夜间的稳态血药浓度进行检测,结果表明其并非维持在一定水平或在一定水平上下波动,而是呈明显的昼夜节律变化,最高峰大多在1AM。国外文献有类似报道〔1-3〕,国内则未见。其机理尚不清楚,被认为可能与5-FU重要分解限速酶二氢尿嘧啶脱氢酶(DPD)的昼夜节律变化有关〔4〕。
本试验中我们发现夜间静滴,5-FU血药浓度及AUC与本方案主要剂量限制性毒性口腔粘膜炎相关,白天静滴无相关性。故认为夜间静滴5-FU,其血药浓度、AUC高低可能是影响口腔粘膜的主要因素,而白天并非如此。骨髓、胃肠道粘膜上皮细胞合成活跃,是5-FU化疗产生毒性最常见组织,有文献报道胃肠道粘膜上皮细胞DNA合成有较明显昼夜节律,早晨比夜晚活跃〔5〕。患者白天口腔粘膜细胞DNA合成活跃,即使在5-FU血药浓度及AUC较低时,也可能造成对口腔粘膜损伤。故我们建议对于24小时恒速静滴患者,检测其24小时AUC值,特别是夜间血药浓度及AUC值,对于调整5-FU剂量,降低毒性意义更大。
, 百拇医药
3.25-FU合理给药方式的推测
本实验结果显示,5-FU夜间静滴时AUC高于白天,高峰血药浓度时间在1AM,而3AM、5AM时5-FU血药浓度与白天无统计学差异。故我们认为在3AM至5AM给予5-FU可获得较低毒副反应,因为这段时间给药既可避免5-FU在1AM时高峰血药浓度对口腔粘膜的毒性,又避免在口腔粘膜细胞,骨髓细胞DNA合成活跃,耐受性差的白天给予较高浓度5-FU。24小时连续静脉给予5-FU毒性低、疗效好,已成为国外普遍采用的给药方式,在晚期肠癌化疗中,随机临床试验已证实与静脉推注相比,能提高有效率〔6,7〕。故我们认为5-FU较合理给药方式应是24小时连续静滴,高峰滴速在3AM至5AM。这与国外文献对5-FU24小时连续静滴,高峰滴速在4AM时辰给药方式相似〔8〕。在本临床研究中,5-FU给药无法做到变速,虽然患者夜间口腔粘膜细胞DNA合成少,耐受性良好,但因有较高的5-FU高峰血药浓度及AUC,使夜间与白天毒副反应无明显差别。随着可携带的电脑调控程序静脉泵在国内的应用,我们将在肠癌、鼻咽癌、乳腺癌等肿瘤化疗中,对5-FU时辰给药方式与连续恒速静滴进行随机对照研究,进一步评价5-FU时辰给药的临床价值。
, http://www.100md.com
作者简介:何友兼 通讯作者
〔参考文献〕
〔1〕Harris BE,Song R, Soong ST, et al. Relationship between dihydropyrimidine dehydrogenase activity and plasma 5-fluorouracil levels with evidence for circadian variation of enzyme activity and plasma drug levels in cancer patients receiving 5-fluorouracil by protracted continuous infusion〔J〕.Cancer Res,1990,50:197~201.
〔2〕Metzger G,Massari C, Etienne MC, et al. Spontaneous or imposed circadian changes in plasma concentrations of 5-fluorouracil coadministered with folinic acid and oxaliplatin:relationship with mucosal toxicity in patients with cancer〔J〕.Clin Pharmacol Ther,1994, 56:190~201.
, 百拇医药
〔3〕Petit E,Milano G, Levi F,et al. Circadian rhythm-varying plasma concentration of 5-fluorouracil during a five day continuous infusion at a constant rate in cancer patients〔J〕.Cancer Res,1988,48:1676~1679.
〔4〕Hrushesky WJ, Bjarnason GA,Circadian cancer therapy〔J〕.J Clin Oncol,1993,11:1403~1417.
〔5〕Buchi KN,Moore JG, Hrushesky WJ,et al.Circadian rhythm of cellular proliferation in the human rectal mucosa 〔J〕.Gastroenterology,1991,101:410~415.
, http://www.100md.com
〔6〕Lokich JJ,Ahlgren JD,Gullo JJ,et al.A prospective randomized comparison of continous infusion fluorouracil with a conventional bolus schedule in metastatic colorectal carcinoma:a Mid-Atlantic Oncology Program Study 〔J〕.J Clin Oncol, 1989,7:425~432.
〔7〕Weinerman B,Shah A,Fields A,et al.A randomized trail of continous systemic infusion verus bolus therapy with 5-fluorouracil in metastatic measurable colorectal cancer〔J〕.Proc Am Soc Clin Oncol,1991,10:143.
〔8〕Levi F, Misset JL,Brienza S,et al.A chronopharmacologic phase II clinical trail with 5-fluorouracil,folinic acid and oxaliplatin using an ambulatory mutichannal programmable pump. High antitumor effetivaness against metastatic colorectal cancer 〔J〕. Cancer, 1992,69:893~900.
收稿日期:1998-08-11;修回日期:1998-10-21, http://www.100md.com