瑞格列奈治疗2型糖尿病68例疗效观察
作者:陆树常 祝兆德 沈建瑾 杜晓东
单位:祝兆德(建德 311612 建德市第三人民医院);陆树常 沈建瑾 杜晓东(杭州 310004 杭州市红十字会医院)
关键词:2型糖尿病;瑞格列奈
摘要 目的
摘要 目的:观察瑞格列奈降低2型糖尿病人的空腹血糖,餐后2h血糖的 作用。方法:2型糖尿病人68例,随机分为治疗组34例(男18,女16)及对 照组34例(男17,女17)。采用瑞格列奈0.5mg po tid×4周。 结果:能有效降低2型糖尿病人的空腹血糖,餐后2h血糖的作用 。有非常显著性差 异(P<0.01),尚能降低糖化血红蛋白(P<0.01)。结论:瑞格列奈能有效降低2型糖尿病人的空腹血糖,餐后2h血糖,降低血脂 及糖化血红蛋白,且无明显副作用。
, 百拇医药 Efficacy observations of repaglinide in the
treatment of type 2 diabetes among 68 cases
Lu Shuchang
(Hangzhou Municipal Red-Cross Hospital, Hangzhou 310004)
Zhu Zhaode
(Hangzhou Municipal Red-Cross Hospital, Hangzhou 310004)
Shen Jianjin
(Hangzhou Municipal Red-Cross Hospital, Hangzhou 310004)
, 百拇医药
Du Xiaodong
(Hangzhou Municipal Red-Cross Hospital, Hangzhou 310004)
ABSTRACT OBJECTIVE:To evaluate the therapeu tic effectiveness of repaglinide in lowering the FBG and PPBG in type 2 diabetes among 68 patients.METHODS:68 cases were divided at random into two groups:Repaglinide group(34 patients, 17female, 17male) and Placebo-controlled gr oup(34 patients,16female and 18male).0.5mg repaglinide was taken orally before e ach meal in therapeutic group for 4 weeks,while the cases in control were treate d by placebo.RESULTS:Repaglinide could effectively decrease the leve ls of FBG,PPBG and HbA1C with significant difference when compare with the place bo(P<0.01).At the meantime,it could also lower the Cholesterol and triglyceride levels when compared with the control group(P<0.01).CO NCLUSION:Repaglinide was effective in lowering the levels of FBG,PPBG and HbA1C in type 2 diabetes patients without obvious side effects.
, 百拇医药
KEY WORDS type 2 diabetes,repaglinide
Repaglinide is a derivative of benzoic acid.Its effect is mediated by inhibiting ATP-dependent potassium ion channels in the plasma membrane of the B-cell [1].The decreased potassium efflux depolarizes the B-cell membrane and open s voltage-dependent calcium ion channels,thus increases the influx of calcium i ons,finally induces insulin release.Repaglinide differs from the biguanide and t he sulphonylureas(SUs)[2].Secondly,there are at least two different aspe cts:①Repaglinide has no sulfo in its chemical structure.②Repaglinide regulates the ATP-sensitive potassium channel via a different binding site on the B-cel l.Repaglinide has a short duration of action and can quickly induce the release of insulin to decrease FBG and PPBG to treat the meal-related PPBG.In the prese nt study,68 type 2 diabetes patients met the requirement of WHO Diabetes Diagnos tic Standard 1985.They were randomly divided evenly into two groups:Repaglinide group and Placebo group.
, 百拇医药
There were 18 male and 16 female patients in the Repaglinide group with the aver age age of 59.1±6.3 and mean duration of diabetes of 3.2±1.2 years,while in t he.Placebo group there were 17 male and 17 female patients,with the average age of 58.9±6.3 and the mean duration of diabetes of 3.3±1.1 years.Among all subje cts,61 patients have never used any OHA and insulin.They have been controlled by diet only.The other 7 patients had the history of OHA treatment but had stopped at least half month before the study.
, http://www.100md.com
Methods
All the patients must have a two-week wash-out period before initiating any tr eatment.During they received FBG and PPBG examination once a week.The patients w hose blood sugar is stable which means the difference between two measurements i s less than 20% would be randomized.Then patients in repaglinide group were give n 0.5mg repaglinide(NovoNorm,Novo Nordisk Denmark) which were taken orally with the main meal three times a day.The treatment lasted for 4 weeks.The other group were cotrolled by diet only.
, 百拇医药
Asseassments:
All the patients were tested as the flow chart.Two groups were compared with eac h other.
-2/week
0/ru n-in
2
4
FBG
*
*
*
*
, 百拇医药
PPBG
*
*
*
*
FBI
*
*
PPBI
*
*
HbA1C
*
*
, 百拇医药
Cholesterol
*
*
Ttiglyceride
*
*
Statistic
All the data is expressed as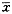 ±S and the difference between before and after treament was examined by par-T test.
±S and the difference between before and after treament was examined by par-T test.
, http://www.100md.com
Results:
General information:
Before treatment, The MBI of patients in repaglinide and placebo-controlled gro up is 26.13±0.55 and 24.81±0.43 respectively before treatment,and 26.01±0.51 and 24.31±0.33 respectively after treatment.There was no significant difference between them.
Clinical Efficiency:
See fig 1
In repaglinide group,blood lervel of cholesterol lowered remarkable from (6.90± 0.21)mmol/L after treatment,and have siguinf cant difference(P<0.01 ) compared with controlled group,so did with triglyceride
, http://www.100md.com
Fig 1 The variation of Blood Sugar,insulin and HbA1C before and after treatment/n=34
Repaglinide/Week0
Placepo-Controll ed/Week
0
2
4
0
2
4
FBG/mmol.L-1
, 百拇医药
10.53±0.37
8.4±0.53*1,*2
7.56±0.30* 1,*2
10.18±0.58
9.15±0.45
9.18±0.44
PPBG/mmol.L-1
13.7±0.45
10.51±0.33*1,*2
8.86±0 .32*1,*2
, 百拇医药
13.6±0.45
12.6±0.34
12.8±0.46
FBI Miu/L
6.66±0.87
7.01±1.65
6.68±0.88
6.7 2±0.71
PPBI Miu/L
46.32±3.18
86.81±5019
46.44±3.23
, 百拇医药
55.86±4.12
HbA1C/%
9.82±0.35
8.16±0.41
9.96 ±0.41
9085±0.51
Compared with pre and post treatmrnt,*1P<0.01;Compared w ith two groups,P<0.01
[from (3.16±0.04)mmol/L to (2.1±0.02)mmol/L,P<0.01].There were no kidney and liver dysfuction found during the trial.
, 百拇医药
Side Effect:
Mild hypoglycemia is reported only in one patient(Blood sugar is 4.2mmol/L).
The symptom of hypoglycemia was relieved after patients taking appropriate solut ion.This phenomena never happened later in his treatment.
Discussion:
Repaglinide is a new type of OHA.It has notable effect on decreasing FBG and PPB G.The mechanism is to stimulate insulin secretion[3,4].The reason of FBG ′s decreasing may contribute to the target cell′s sensitivity to insulin incrc asing because of the whole blood sugar control.Along with the treatment,area und er the curve(AUC) of 24 hours blood glucose profile diminished,and HbA1C falled [5].The improvement of the lipoprorein may also relate to whole blood su gar control.
, http://www.100md.com
As more and more age diadetics come,patients often suffer from liver and kidney dysfunction and hypoglycemic.NovoNorm is a suitable OHA for these patients since repaglieide has a short duration of action.It decrease the incidence of hypogly cemia to a minimum[6].NovoNorm is a safe and efficient OHA.
Reference
1,Owens DR.Repaglinide-prandial glucose regulator: a new class of oral antidiabetic drug.Diabet Med,1998,15(Suppl 4)∶s28.
, http://www.100md.com
2,Wolffenbuttel BHR,Nijst L,Sels JPLE,et al.Effecte of a new oral hypoglycaemic agent,repaglinide,on metabolic control in sulphonylurea-treated patients with NIDDM.Eur J Clin Pharmaco,1993,45∶113.
3,Owens DR,Donockley W,Robling MR,et al .A does-response study of a new(non-sulphonylurea) agentin diet-reated patien ts with non-insulin-dependent diabetes mellitus(NIDDM).5 th World conference o n Clinical Pharmacology and Therapeutics,Yokohama,Japan,1992.
, 百拇医药
4,Robling MR,Dolben J,Luzio SD,et al.Si ngle dose-response study of a new oral hypoglycaemic agent in diet-treated pat ients with non-insulin-dependent diabetes mellitus(NIDDM).Br J Clin Pharmacol, 1992,34∶173.
5,Koevary SB.Effects of acarbose on the developmen of diabetes and its renal complications in BB/Wor rat.Drugs in Development.p 189 -197.Vol 1.a-Glucosidase lnhibition.Potential Use in Diabetes.edited by JR Vass elli,CA Maggio,and A Scriabine.1993 NervPress.Brantord.Connectcut,USA.
6,Tronier B,Marbury TC,Damsbo P,et al.A new oralhypoglycaemic agent,repaglinide,minimises risk of hypoglycaemia in well -controlled NIDDM patients.Diabetologia,1995,38∶Abstract 725., 百拇医药
单位:祝兆德(建德 311612 建德市第三人民医院);陆树常 沈建瑾 杜晓东(杭州 310004 杭州市红十字会医院)
关键词:2型糖尿病;瑞格列奈
摘要 目的
摘要 目的:观察瑞格列奈降低2型糖尿病人的空腹血糖,餐后2h血糖的 作用。方法:2型糖尿病人68例,随机分为治疗组34例(男18,女16)及对 照组34例(男17,女17)。采用瑞格列奈0.5mg po tid×4周。 结果:能有效降低2型糖尿病人的空腹血糖,餐后2h血糖的作用 。有非常显著性差 异(P<0.01),尚能降低糖化血红蛋白(P<0.01)。结论:瑞格列奈能有效降低2型糖尿病人的空腹血糖,餐后2h血糖,降低血脂 及糖化血红蛋白,且无明显副作用。
, 百拇医药 Efficacy observations of repaglinide in the
treatment of type 2 diabetes among 68 cases
Lu Shuchang
(Hangzhou Municipal Red-Cross Hospital, Hangzhou 310004)
Zhu Zhaode
(Hangzhou Municipal Red-Cross Hospital, Hangzhou 310004)
Shen Jianjin
(Hangzhou Municipal Red-Cross Hospital, Hangzhou 310004)
, 百拇医药
Du Xiaodong
(Hangzhou Municipal Red-Cross Hospital, Hangzhou 310004)
ABSTRACT OBJECTIVE:To evaluate the therapeu tic effectiveness of repaglinide in lowering the FBG and PPBG in type 2 diabetes among 68 patients.METHODS:68 cases were divided at random into two groups:Repaglinide group(34 patients, 17female, 17male) and Placebo-controlled gr oup(34 patients,16female and 18male).0.5mg repaglinide was taken orally before e ach meal in therapeutic group for 4 weeks,while the cases in control were treate d by placebo.RESULTS:Repaglinide could effectively decrease the leve ls of FBG,PPBG and HbA1C with significant difference when compare with the place bo(P<0.01).At the meantime,it could also lower the Cholesterol and triglyceride levels when compared with the control group(P<0.01).CO NCLUSION:Repaglinide was effective in lowering the levels of FBG,PPBG and HbA1C in type 2 diabetes patients without obvious side effects.
, 百拇医药
KEY WORDS type 2 diabetes,repaglinide
Repaglinide is a derivative of benzoic acid.Its effect is mediated by inhibiting ATP-dependent potassium ion channels in the plasma membrane of the B-cell [1].The decreased potassium efflux depolarizes the B-cell membrane and open s voltage-dependent calcium ion channels,thus increases the influx of calcium i ons,finally induces insulin release.Repaglinide differs from the biguanide and t he sulphonylureas(SUs)[2].Secondly,there are at least two different aspe cts:①Repaglinide has no sulfo in its chemical structure.②Repaglinide regulates the ATP-sensitive potassium channel via a different binding site on the B-cel l.Repaglinide has a short duration of action and can quickly induce the release of insulin to decrease FBG and PPBG to treat the meal-related PPBG.In the prese nt study,68 type 2 diabetes patients met the requirement of WHO Diabetes Diagnos tic Standard 1985.They were randomly divided evenly into two groups:Repaglinide group and Placebo group.
, 百拇医药
There were 18 male and 16 female patients in the Repaglinide group with the aver age age of 59.1±6.3 and mean duration of diabetes of 3.2±1.2 years,while in t he.Placebo group there were 17 male and 17 female patients,with the average age of 58.9±6.3 and the mean duration of diabetes of 3.3±1.1 years.Among all subje cts,61 patients have never used any OHA and insulin.They have been controlled by diet only.The other 7 patients had the history of OHA treatment but had stopped at least half month before the study.
, http://www.100md.com
Methods
All the patients must have a two-week wash-out period before initiating any tr eatment.During they received FBG and PPBG examination once a week.The patients w hose blood sugar is stable which means the difference between two measurements i s less than 20% would be randomized.Then patients in repaglinide group were give n 0.5mg repaglinide(NovoNorm,Novo Nordisk Denmark) which were taken orally with the main meal three times a day.The treatment lasted for 4 weeks.The other group were cotrolled by diet only.
, 百拇医药
Asseassments:
All the patients were tested as the flow chart.Two groups were compared with eac h other.
-2/week
0/ru n-in
2
4
FBG
*
*
*
*
, 百拇医药
PPBG
*
*
*
*
FBI
*
*
PPBI
*
*
HbA1C
*
*
, 百拇医药
Cholesterol
*
*
Ttiglyceride
*
*
Statistic
All the data is expressed as
, http://www.100md.com
Results:
General information:
Before treatment, The MBI of patients in repaglinide and placebo-controlled gro up is 26.13±0.55 and 24.81±0.43 respectively before treatment,and 26.01±0.51 and 24.31±0.33 respectively after treatment.There was no significant difference between them.
Clinical Efficiency:
See fig 1
In repaglinide group,blood lervel of cholesterol lowered remarkable from (6.90± 0.21)mmol/L after treatment,and have siguinf cant difference(P<0.01 ) compared with controlled group,so did with triglyceride
, http://www.100md.com
Fig 1 The variation of Blood Sugar,insulin and HbA1C before and after treatment/n=34
Repaglinide/Week0
Placepo-Controll ed/Week
0
2
4
0
2
4
FBG/mmol.L-1
, 百拇医药
10.53±0.37
8.4±0.53*1,*2
7.56±0.30* 1,*2
10.18±0.58
9.15±0.45
9.18±0.44
PPBG/mmol.L-1
13.7±0.45
10.51±0.33*1,*2
8.86±0 .32*1,*2
, 百拇医药
13.6±0.45
12.6±0.34
12.8±0.46
FBI Miu/L
6.66±0.87
7.01±1.65
6.68±0.88
6.7 2±0.71
PPBI Miu/L
46.32±3.18
86.81±5019
46.44±3.23
, 百拇医药
55.86±4.12
HbA1C/%
9.82±0.35
8.16±0.41
9.96 ±0.41
9085±0.51
Compared with pre and post treatmrnt,*1P<0.01;Compared w ith two groups,P<0.01
[from (3.16±0.04)mmol/L to (2.1±0.02)mmol/L,P<0.01].There were no kidney and liver dysfuction found during the trial.
, 百拇医药
Side Effect:
Mild hypoglycemia is reported only in one patient(Blood sugar is 4.2mmol/L).
The symptom of hypoglycemia was relieved after patients taking appropriate solut ion.This phenomena never happened later in his treatment.
Discussion:
Repaglinide is a new type of OHA.It has notable effect on decreasing FBG and PPB G.The mechanism is to stimulate insulin secretion[3,4].The reason of FBG ′s decreasing may contribute to the target cell′s sensitivity to insulin incrc asing because of the whole blood sugar control.Along with the treatment,area und er the curve(AUC) of 24 hours blood glucose profile diminished,and HbA1C falled [5].The improvement of the lipoprorein may also relate to whole blood su gar control.
, http://www.100md.com
As more and more age diadetics come,patients often suffer from liver and kidney dysfunction and hypoglycemic.NovoNorm is a suitable OHA for these patients since repaglieide has a short duration of action.It decrease the incidence of hypogly cemia to a minimum[6].NovoNorm is a safe and efficient OHA.
Reference
1,Owens DR.Repaglinide-prandial glucose regulator: a new class of oral antidiabetic drug.Diabet Med,1998,15(Suppl 4)∶s28.
, http://www.100md.com
2,Wolffenbuttel BHR,Nijst L,Sels JPLE,et al.Effecte of a new oral hypoglycaemic agent,repaglinide,on metabolic control in sulphonylurea-treated patients with NIDDM.Eur J Clin Pharmaco,1993,45∶113.
3,Owens DR,Donockley W,Robling MR,et al .A does-response study of a new(non-sulphonylurea) agentin diet-reated patien ts with non-insulin-dependent diabetes mellitus(NIDDM).5 th World conference o n Clinical Pharmacology and Therapeutics,Yokohama,Japan,1992.
, 百拇医药
4,Robling MR,Dolben J,Luzio SD,et al.Si ngle dose-response study of a new oral hypoglycaemic agent in diet-treated pat ients with non-insulin-dependent diabetes mellitus(NIDDM).Br J Clin Pharmacol, 1992,34∶173.
5,Koevary SB.Effects of acarbose on the developmen of diabetes and its renal complications in BB/Wor rat.Drugs in Development.p 189 -197.Vol 1.a-Glucosidase lnhibition.Potential Use in Diabetes.edited by JR Vass elli,CA Maggio,and A Scriabine.1993 NervPress.Brantord.Connectcut,USA.
6,Tronier B,Marbury TC,Damsbo P,et al.A new oralhypoglycaemic agent,repaglinide,minimises risk of hypoglycaemia in well -controlled NIDDM patients.Diabetologia,1995,38∶Abstract 725., 百拇医药