肝细胞水化状态对细胞某些代谢的影响
作者:赵利斌 郝飞
单位:赵利斌(第三军医大学附属西南医院感染病科、全军传染病专科中心,重庆 400038);郝飞(第三军医大学附属西南医院感染病科、全军传染病专科中心,重庆 400038)
关键词:肝细胞水化状态;代谢影响
第三军医大学学报000526 提 要: 目的 探讨不等渗条件下肝细胞水化状态改变对某些代谢的影响。方法 动态观察培养鼠肝细胞[3H]亮氨酸、[3H]TdR掺入量,肝细胞膜Na+/K+-ATP酶及上清液中ALT、AST、LDH活性。结果 培养120 min,与等渗组比较,高渗组肝细胞[3H]亮氨酸和[3H]TdR掺入量分别减少72.99%和65.70%(P<0.001),低渗组减少15.94%和10.41%(P>0.05)。高渗组肝细胞膜Na+/K+-ATP酶活性增高,低渗组降低,与等渗组比较差异显著(P<0.05与P<0.01)。肝细胞膜Na+/K+-ATP酶活性与[3H]掺入量在低渗组呈非常显著负相关,高渗组呈非常显著正相关(P<0.001)。培养上清液ALT、AST、LDH活性在正常范围内波动。结论 肝细胞水化状态可作为影响肝细胞代谢的触发因素,而Na+/K+-ATP酶活性改变可能是这一状态下细胞代谢调节机制之一。
, http://www.100md.com
中图法分类号: R322.47;R329.2 文献标识码: A
文章编号:1000-5404(2000)05-0490-03
Effects of hepatocellular hydration state on certain kinds of hepatocellular metabolism
ZHAO Li-bin, HAO Fei
(Clinical Center of Infectious Disease, Southwest Hospital, Third Military Medical University, Chongqing 400038,China)
Abstract: Objective To observe certain metabolic changes induced by alterations of hepatocellular hydration state under aniso-osmotic condition. Methods Incorporating rate of [3H] leucine into protein and that of [3H]TdR into DNA were measured and the activity of Na+/K+-ATPase of hepatocellular membrane and that of ALT, AST and LDH in the supernatant of cell culture observed dynamically. Results After the cells were cultured for 120 min, the incorporating rates of [3H] leucine and [3H]TdR in the hepatocytes of hyper-osmotic group were significantly inhibited as compared with the normo-osmotic and hypo-osmotic groups (P<0.01 and 0.05). These two rates were closely correlated in all the 3 groups (P<0.001). The activity of Na+/K+-ATPase in the hyper-osmotic group was markedly increased but that in the hypo-osmotic group decreased as compared with that in the normo-osmotic group (P<0.05 and 0.01). The two incorporating rates in the hypo-osmotic group were negatively but those in the hyper-osmotic group positively correlated to the activity of Na+/K+-ATPase. The activity of ALT, AST and LDH in the supernatant of cell culture was within the normal range. Conclusion The changes in hepatocellular hydration state may be one of the physiological factors to modulate hepatocellular metabolism, in which the activity of Na+/K+-ATPase of hepatocellular membrane might regulate the levels of protein and DNA synthesis under this condition.
, 百拇医药
Key words: hepatocellular hydration state; metabolic modulation
近年来研究提示,细胞水化状态(即细胞容积)与细胞功能状态密切相关,故有关细胞水化状态对细胞功能的影响及其意义引人注目。肝细胞水化状态,作为一种触发信号,引发蛋白等一系列代谢改变[1~3]。但是,肝细胞水化状态变化导致膜Na+/K+-ATP酶活性的变化规律以及对细胞内蛋白和DNA合成的影响尚不清楚。本实验旨在通过观察肝细胞水化状态变化后,对膜Na+/K+-ATP酶活性及蛋白、DNA合成的影响,以探讨肝细胞水化状态调节细胞代谢的机制。
1 材料与方法
1.1 鼠肝细胞分离
雄性Wistar大鼠(180~200 g),用体外肝细胞灌流法[4]分离纯化鼠肝细胞,活性90%以上,预培养20 h。分别换210、320、450 m0sm培养基(作[3H]掺入的培养基中含[3H]20 μCi/ml或740 kBq)。用超微量渗量计核准渗透压,于培养30、60、90、120 min进行检测,等渗(320 m0sm)培养组所测指标作对照组。
, http://www.100md.com
1.2 肝细胞容积测定
用F-800全自动血细胞计数仪测定,以f1表示。
1.3 [3H]亮氨酸与[3H]TdR掺入
参考Stoll等[5]的方法采集[3H]掺入的标本,LKB 1217型液体闪烁计数器测定[3H]掺入的每分衰变率,再换算为[3H]亮氨酸与[3H]TdR掺入每毫克蛋白或DNA的飞摩尔(fmol/mg)。
1.4 膜Na+/K+-ATP酶活性测定
按文献[6]方法提取培养肝细胞膜后,用钼蓝比法测定Na+/K+-ATP酶活性[7],酶活性单位以nmol.mg-1.min-1表示。
, 百拇医药
1.5 ALT、AST、LDH活性测定
CX-全自动生化分析仪测定同期培养上清液中ALT、AST和LDH活性。测定等渗培养鼠肝细胞上清液中各种酶活性的平均值并参考上述生化仪所提供的正常参数范围作正常值。
2 结果
2.1 肝细胞容积测定
肝细胞容积在低渗组最大,高渗组最小,与等渗组比较差异非常显著(P<0.001),见表。
表1 不同渗透压培养鼠肝细胞容积改变(f1)
Tab 1 The changes of hepatocyte volumes of rats under
aniso-osmotic conditions(fl)
, http://www.100md.com
Hepatocyte
volume
t
Hypo-osmotic
(210 mOsm)
121.4±2.8***
40.67
Normo-osmotic
(320 mOsm)
78.4±4.2
Hyper-osmotic
, http://www.100md.com
(450 mOsm)
61.3±2.9***
12.98
***:P<0.001 vs normo-osmotic
2.2 [3H]亮氨酸与[3H]TdR DNA掺入量
随培养时间延长掺入量呈曲线增加。与对照组比较,培养120 min后,高渗组分别减少72.99%与65.70%(P<0.05,P<0.001);低渗组减少15.94%与10.41%(P>0.05),见表2、表3。
表2 不同渗透压培养鼠肝细胞[3H]亮氨酸掺入量(fmol/mg,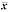 ±s)
±s)
, http://www.100md.com
Tab 2 The incorporation rates of [3H] leucine in the hepatocytes
under aniso-osmotic conditions in rats(fmol/mg,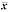 ±s)
±s)
Culture time(min)
30
60
90
120
Hypo-osmotic
(210 mOsm)
, http://www.100md.com
3.15±0.59
3.89±0.41
5.17±0.67
3.82±2.43
Normo-osmotic
(320 mOsm)
3.85±0.93
4.46±1.32
5.63±1.47
16.44±1.82
Hyper-osmotic
, 百拇医药
(450 mOsm)
1.92±0.24***
2.45±0.28**
3.55±0.82*
4.44±1.10***
*:P<0.05),**:P<0.01,***:P<0.001 vs normo-osmotic
表3 不同渗透压培养鼠肝细胞[3H]TdR DNA掺入量(fmol/mg,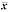 ±s)
±s)
Tab 3 The incorporation rates of [3H] TdR DNA in the
, http://www.100md.com
hepatocytes under aniso-osmotic conditions in rats (fmol/mg,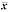 ±s)
±s)
Culture time(min)
30
60
90
120
Hypo-osmotic
(210 mOsm)
4.42±1.07
5.39±0.64
, http://www.100md.com
7.18±1.26
11.10±2.00
Normo-osmotic
(320 mOsm)
4.82±0.59
5.93±0.82
8.02±0.87
12.39±1.96
Hyper-osmotic
(450 mOsm)
2.36±0.68***
, 百拇医药
3.40±0.83***
3.65±0.78***
4.25±1.0***
***:P<0.001 vs normo-osmotic
对不同渗透压培养鼠肝细胞[3H]亮氨酸与[3H]TdR蛋白与DNA的掺入量进行相关性分析,每组24例,r值在低渗组为0.9456,等渗组为0.9475,高渗组为0.8516,均呈非常显著正相关(P<0.001)。
2.3 不同渗透压培养鼠肝细胞膜Na+/K+-ATP酶活性测定
等渗组肝细胞膜Na+/K+-ATP酶活性在92.71~95.00 nmolmg-1.min-1间波动,低渗组随时间延长逐渐降低为88.17~75.00 nmol.mg-1.min-1,高渗组逐渐升高为97.17~107.33 nmol.mg-1.min-1,培养60~120 min与等渗组比较均差异显著(P<0.001,P<0.01和P<0.05)。
, 百拇医药
2.4 肝细胞膜Na+/K+-ATP酶活性与[3H]亮氨酸、[3H]TdR掺入量的相关性分析,见表4
表4 不同渗透压培养鼠肝细胞膜Na+/K+-ATP酶活性与
[3H]亮氨酸、[3H]TdR掺入量的相关性分析(n=24)
Tab 4 The relative analysis among the in corporation rates
of [3H] leucine, [3H] TdR DNA of the hepatocytes and
, 百拇医药
the activity of Na+/K+-ATPase of hepatocyte membrane (n=24)
Incorporation of
[3H]leucine
and Na+/K+-ATPase
Incorporation of
[3H]TdR
and Na+/K+-ATPase
Hypo-osmotic
, 百拇医药
(210 mOsm)
-0.6920***
-0.5798**
Normo-osmotic
(320 mOsm)
0.3351
0.4684
Hyper-osmotic
(450 mOsm)
0.8328***
0.9108***
, 百拇医药
**:P<0.01,***:P<0.001 vs normo-osmotic
2.5 培养上清液中ALT、AST、LDH活性变化
ALT、AST活性正常值为6~45 IU/L,LDH活性为110~24.0 U/L,3种酶活性在整个培养期内始终在正常临界范围波动。
3 讨论
研究认为,肝细胞水化状态是控制蛋白等一系列物质分解的重要因素,细胞肿胀时抑制蛋白分解并激活合成,而皱缩时则完全相反[1,8]。本研究在证实肝细胞水化状态变化的基础上,用[3H]掺入法了解肝细胞蛋白与DNA掺入的改变。结果显示,在培养期内(120 min)[3H]蛋白和DNA掺入量以等渗组最高。与等渗组比较,低渗组轻度减少(P>0.05),而高渗组显著减少(P<0.001),与其他学者报道较为接近[5],肝细胞水化状态诱发的[3H]亮氨酸[3H]TdR掺入量改变之间呈非常显著正相关,提示肝细胞水化状对细胞内蛋白和DNA合成的影响是完全一致的。
, http://www.100md.com
肝细胞水化状态使肝细胞膜Na+/K+-ATP酶活性改变的规律及与DNA和蛋白合成关系尚不清楚。肝细胞容积改变后膜Na+/K+-ATP酶活性测定发现,随培养时间延长与等渗组比较,高渗组膜酶活性逐渐增高,低渗组则随时间延长逐渐降低。将膜酶活性与[3H]亮氨酸蛋白掺入量和[3H]TdR DNA掺入量作相关分析发现,低渗时两者呈非常显著负相关(P<0.01),而高渗时呈非常显著正相关(P<0.001),说明高渗时膜Na+/K+-ATP酶激活,消耗细胞内能量,从而影响细胞内蛋白和DNA合成,而低渗时膜酶活性受抑制,可能对细胞内蛋白和DNA合成有利。但是Na+/K+-ATP酶活性改变是否通过其他机制,直接或间接地影响细胞内蛋白或DNA合成尚有待进一步研究。
培养上清液中反映肝细胞损伤的指标ALT、AST、LDH活性始终在正常临界范围波动,说明肝细胞肿胀膜通透性增高,酶溢出略增加并非肝细胞病理性损害。也进一步验证不同渗透压培养对鼠肝细胞蛋白和DNA合成抑制及膜酶活性变化受细胞水化状态调节,且这一改变属生理性[1]。
, http://www.100md.com
作者简介:赵利斌(1953-),女,湖北省宜昌市人,副主任医师,主要从事肝炎的诊断与治疗方面的研究,现在解放军第161中心医院,武汉 430010。电话:(023)68754000-73080
参考文献:
[1] Haussinger D, Schliess F. Cell volume and hepatocellular function[J]. J Hepatol,1995,22(1):94-100.
[2] Agius L, Peak M, Beresford G, et al. The role of ion content and cell volume in insulin action[J]. Biochem Soc Trans,1994,22(3):516-522.
[3] Haussinger D, Roth E, Lang F, et al. Cellular hydration state: an important determinant of protein catabolism in health and disease[J]. Lancet,1993,341(8 856):1 330-1 332.
, 百拇医药
[4] 王宇明,陈国致,董家鸿,等.分离肝细胞的一种体外灌流法[J].中华消化杂志,1994,14(3):175.
[5] Stoll B, Gerok W, Land F, et al. Liver cell volume and protein synthesis[J]. Biochem J,1992,287(1):217-222.
[6] 孙志贤 主编.现代生物学理论与研究技术[M].北京:军事医学科学出版社,1995:300.
[7] Unverferth D V, Lee S W, Wallick K T. Human myocardial adenosine triphosphatase activities in health and heart failure[J]. Am Heart J, 1988,115(1):139-146.
, 百拇医药
[8] Holen L, Gordon P B, Seglen P O. Inhibition of hepatocytic autophagy by okadaic acid and other protein phosphatase inhibitors[J]. Eur J Biochem,1993,215(1):113-122.
[9] Haussinger D, Lang F, Gerok W. Regulation of cell function by the celluar hydration state[J]. Am J Physiol,1994,267(3):E343-355.
收稿日期:1998-12-05;修回日期:1999-09-21, 百拇医药
单位:赵利斌(第三军医大学附属西南医院感染病科、全军传染病专科中心,重庆 400038);郝飞(第三军医大学附属西南医院感染病科、全军传染病专科中心,重庆 400038)
关键词:肝细胞水化状态;代谢影响
第三军医大学学报000526 提 要: 目的 探讨不等渗条件下肝细胞水化状态改变对某些代谢的影响。方法 动态观察培养鼠肝细胞[3H]亮氨酸、[3H]TdR掺入量,肝细胞膜Na+/K+-ATP酶及上清液中ALT、AST、LDH活性。结果 培养120 min,与等渗组比较,高渗组肝细胞[3H]亮氨酸和[3H]TdR掺入量分别减少72.99%和65.70%(P<0.001),低渗组减少15.94%和10.41%(P>0.05)。高渗组肝细胞膜Na+/K+-ATP酶活性增高,低渗组降低,与等渗组比较差异显著(P<0.05与P<0.01)。肝细胞膜Na+/K+-ATP酶活性与[3H]掺入量在低渗组呈非常显著负相关,高渗组呈非常显著正相关(P<0.001)。培养上清液ALT、AST、LDH活性在正常范围内波动。结论 肝细胞水化状态可作为影响肝细胞代谢的触发因素,而Na+/K+-ATP酶活性改变可能是这一状态下细胞代谢调节机制之一。
, http://www.100md.com
中图法分类号: R322.47;R329.2 文献标识码: A
文章编号:1000-5404(2000)05-0490-03
Effects of hepatocellular hydration state on certain kinds of hepatocellular metabolism
ZHAO Li-bin, HAO Fei
(Clinical Center of Infectious Disease, Southwest Hospital, Third Military Medical University, Chongqing 400038,China)
Abstract: Objective To observe certain metabolic changes induced by alterations of hepatocellular hydration state under aniso-osmotic condition. Methods Incorporating rate of [3H] leucine into protein and that of [3H]TdR into DNA were measured and the activity of Na+/K+-ATPase of hepatocellular membrane and that of ALT, AST and LDH in the supernatant of cell culture observed dynamically. Results After the cells were cultured for 120 min, the incorporating rates of [3H] leucine and [3H]TdR in the hepatocytes of hyper-osmotic group were significantly inhibited as compared with the normo-osmotic and hypo-osmotic groups (P<0.01 and 0.05). These two rates were closely correlated in all the 3 groups (P<0.001). The activity of Na+/K+-ATPase in the hyper-osmotic group was markedly increased but that in the hypo-osmotic group decreased as compared with that in the normo-osmotic group (P<0.05 and 0.01). The two incorporating rates in the hypo-osmotic group were negatively but those in the hyper-osmotic group positively correlated to the activity of Na+/K+-ATPase. The activity of ALT, AST and LDH in the supernatant of cell culture was within the normal range. Conclusion The changes in hepatocellular hydration state may be one of the physiological factors to modulate hepatocellular metabolism, in which the activity of Na+/K+-ATPase of hepatocellular membrane might regulate the levels of protein and DNA synthesis under this condition.
, 百拇医药
Key words: hepatocellular hydration state; metabolic modulation
近年来研究提示,细胞水化状态(即细胞容积)与细胞功能状态密切相关,故有关细胞水化状态对细胞功能的影响及其意义引人注目。肝细胞水化状态,作为一种触发信号,引发蛋白等一系列代谢改变[1~3]。但是,肝细胞水化状态变化导致膜Na+/K+-ATP酶活性的变化规律以及对细胞内蛋白和DNA合成的影响尚不清楚。本实验旨在通过观察肝细胞水化状态变化后,对膜Na+/K+-ATP酶活性及蛋白、DNA合成的影响,以探讨肝细胞水化状态调节细胞代谢的机制。
1 材料与方法
1.1 鼠肝细胞分离
雄性Wistar大鼠(180~200 g),用体外肝细胞灌流法[4]分离纯化鼠肝细胞,活性90%以上,预培养20 h。分别换210、320、450 m0sm培养基(作[3H]掺入的培养基中含[3H]20 μCi/ml或740 kBq)。用超微量渗量计核准渗透压,于培养30、60、90、120 min进行检测,等渗(320 m0sm)培养组所测指标作对照组。
, http://www.100md.com
1.2 肝细胞容积测定
用F-800全自动血细胞计数仪测定,以f1表示。
1.3 [3H]亮氨酸与[3H]TdR掺入
参考Stoll等[5]的方法采集[3H]掺入的标本,LKB 1217型液体闪烁计数器测定[3H]掺入的每分衰变率,再换算为[3H]亮氨酸与[3H]TdR掺入每毫克蛋白或DNA的飞摩尔(fmol/mg)。
1.4 膜Na+/K+-ATP酶活性测定
按文献[6]方法提取培养肝细胞膜后,用钼蓝比法测定Na+/K+-ATP酶活性[7],酶活性单位以nmol.mg-1.min-1表示。
, 百拇医药
1.5 ALT、AST、LDH活性测定
CX-全自动生化分析仪测定同期培养上清液中ALT、AST和LDH活性。测定等渗培养鼠肝细胞上清液中各种酶活性的平均值并参考上述生化仪所提供的正常参数范围作正常值。
2 结果
2.1 肝细胞容积测定
肝细胞容积在低渗组最大,高渗组最小,与等渗组比较差异非常显著(P<0.001),见表。
表1 不同渗透压培养鼠肝细胞容积改变(f1)
Tab 1 The changes of hepatocyte volumes of rats under
aniso-osmotic conditions(fl)
, http://www.100md.com
Hepatocyte
volume
t
Hypo-osmotic
(210 mOsm)
121.4±2.8***
40.67
Normo-osmotic
(320 mOsm)
78.4±4.2
Hyper-osmotic
, http://www.100md.com
(450 mOsm)
61.3±2.9***
12.98
***:P<0.001 vs normo-osmotic
2.2 [3H]亮氨酸与[3H]TdR DNA掺入量
随培养时间延长掺入量呈曲线增加。与对照组比较,培养120 min后,高渗组分别减少72.99%与65.70%(P<0.05,P<0.001);低渗组减少15.94%与10.41%(P>0.05),见表2、表3。
表2 不同渗透压培养鼠肝细胞[3H]亮氨酸掺入量(fmol/mg,
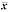 ±s)
±s), http://www.100md.com
Tab 2 The incorporation rates of [3H] leucine in the hepatocytes
under aniso-osmotic conditions in rats(fmol/mg,
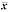 ±s)
±s)Culture time(min)
30
60
90
120
Hypo-osmotic
(210 mOsm)
, http://www.100md.com
3.15±0.59
3.89±0.41
5.17±0.67
3.82±2.43
Normo-osmotic
(320 mOsm)
3.85±0.93
4.46±1.32
5.63±1.47
16.44±1.82
Hyper-osmotic
, 百拇医药
(450 mOsm)
1.92±0.24***
2.45±0.28**
3.55±0.82*
4.44±1.10***
*:P<0.05),**:P<0.01,***:P<0.001 vs normo-osmotic
表3 不同渗透压培养鼠肝细胞[3H]TdR DNA掺入量(fmol/mg,
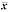 ±s)
±s)Tab 3 The incorporation rates of [3H] TdR DNA in the
, http://www.100md.com
hepatocytes under aniso-osmotic conditions in rats (fmol/mg,
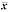 ±s)
±s)Culture time(min)
30
60
90
120
Hypo-osmotic
(210 mOsm)
4.42±1.07
5.39±0.64
, http://www.100md.com
7.18±1.26
11.10±2.00
Normo-osmotic
(320 mOsm)
4.82±0.59
5.93±0.82
8.02±0.87
12.39±1.96
Hyper-osmotic
(450 mOsm)
2.36±0.68***
, 百拇医药
3.40±0.83***
3.65±0.78***
4.25±1.0***
***:P<0.001 vs normo-osmotic
对不同渗透压培养鼠肝细胞[3H]亮氨酸与[3H]TdR蛋白与DNA的掺入量进行相关性分析,每组24例,r值在低渗组为0.9456,等渗组为0.9475,高渗组为0.8516,均呈非常显著正相关(P<0.001)。
2.3 不同渗透压培养鼠肝细胞膜Na+/K+-ATP酶活性测定
等渗组肝细胞膜Na+/K+-ATP酶活性在92.71~95.00 nmolmg-1.min-1间波动,低渗组随时间延长逐渐降低为88.17~75.00 nmol.mg-1.min-1,高渗组逐渐升高为97.17~107.33 nmol.mg-1.min-1,培养60~120 min与等渗组比较均差异显著(P<0.001,P<0.01和P<0.05)。
, 百拇医药
2.4 肝细胞膜Na+/K+-ATP酶活性与[3H]亮氨酸、[3H]TdR掺入量的相关性分析,见表4
表4 不同渗透压培养鼠肝细胞膜Na+/K+-ATP酶活性与
[3H]亮氨酸、[3H]TdR掺入量的相关性分析(n=24)
Tab 4 The relative analysis among the in corporation rates
of [3H] leucine, [3H] TdR DNA of the hepatocytes and
, 百拇医药
the activity of Na+/K+-ATPase of hepatocyte membrane (n=24)
Incorporation of
[3H]leucine
and Na+/K+-ATPase
Incorporation of
[3H]TdR
and Na+/K+-ATPase
Hypo-osmotic
, 百拇医药
(210 mOsm)
-0.6920***
-0.5798**
Normo-osmotic
(320 mOsm)
0.3351
0.4684
Hyper-osmotic
(450 mOsm)
0.8328***
0.9108***
, 百拇医药
**:P<0.01,***:P<0.001 vs normo-osmotic
2.5 培养上清液中ALT、AST、LDH活性变化
ALT、AST活性正常值为6~45 IU/L,LDH活性为110~24.0 U/L,3种酶活性在整个培养期内始终在正常临界范围波动。
3 讨论
研究认为,肝细胞水化状态是控制蛋白等一系列物质分解的重要因素,细胞肿胀时抑制蛋白分解并激活合成,而皱缩时则完全相反[1,8]。本研究在证实肝细胞水化状态变化的基础上,用[3H]掺入法了解肝细胞蛋白与DNA掺入的改变。结果显示,在培养期内(120 min)[3H]蛋白和DNA掺入量以等渗组最高。与等渗组比较,低渗组轻度减少(P>0.05),而高渗组显著减少(P<0.001),与其他学者报道较为接近[5],肝细胞水化状态诱发的[3H]亮氨酸[3H]TdR掺入量改变之间呈非常显著正相关,提示肝细胞水化状对细胞内蛋白和DNA合成的影响是完全一致的。
, http://www.100md.com
肝细胞水化状态使肝细胞膜Na+/K+-ATP酶活性改变的规律及与DNA和蛋白合成关系尚不清楚。肝细胞容积改变后膜Na+/K+-ATP酶活性测定发现,随培养时间延长与等渗组比较,高渗组膜酶活性逐渐增高,低渗组则随时间延长逐渐降低。将膜酶活性与[3H]亮氨酸蛋白掺入量和[3H]TdR DNA掺入量作相关分析发现,低渗时两者呈非常显著负相关(P<0.01),而高渗时呈非常显著正相关(P<0.001),说明高渗时膜Na+/K+-ATP酶激活,消耗细胞内能量,从而影响细胞内蛋白和DNA合成,而低渗时膜酶活性受抑制,可能对细胞内蛋白和DNA合成有利。但是Na+/K+-ATP酶活性改变是否通过其他机制,直接或间接地影响细胞内蛋白或DNA合成尚有待进一步研究。
培养上清液中反映肝细胞损伤的指标ALT、AST、LDH活性始终在正常临界范围波动,说明肝细胞肿胀膜通透性增高,酶溢出略增加并非肝细胞病理性损害。也进一步验证不同渗透压培养对鼠肝细胞蛋白和DNA合成抑制及膜酶活性变化受细胞水化状态调节,且这一改变属生理性[1]。
, http://www.100md.com
作者简介:赵利斌(1953-),女,湖北省宜昌市人,副主任医师,主要从事肝炎的诊断与治疗方面的研究,现在解放军第161中心医院,武汉 430010。电话:(023)68754000-73080
参考文献:
[1] Haussinger D, Schliess F. Cell volume and hepatocellular function[J]. J Hepatol,1995,22(1):94-100.
[2] Agius L, Peak M, Beresford G, et al. The role of ion content and cell volume in insulin action[J]. Biochem Soc Trans,1994,22(3):516-522.
[3] Haussinger D, Roth E, Lang F, et al. Cellular hydration state: an important determinant of protein catabolism in health and disease[J]. Lancet,1993,341(8 856):1 330-1 332.
, 百拇医药
[4] 王宇明,陈国致,董家鸿,等.分离肝细胞的一种体外灌流法[J].中华消化杂志,1994,14(3):175.
[5] Stoll B, Gerok W, Land F, et al. Liver cell volume and protein synthesis[J]. Biochem J,1992,287(1):217-222.
[6] 孙志贤 主编.现代生物学理论与研究技术[M].北京:军事医学科学出版社,1995:300.
[7] Unverferth D V, Lee S W, Wallick K T. Human myocardial adenosine triphosphatase activities in health and heart failure[J]. Am Heart J, 1988,115(1):139-146.
, 百拇医药
[8] Holen L, Gordon P B, Seglen P O. Inhibition of hepatocytic autophagy by okadaic acid and other protein phosphatase inhibitors[J]. Eur J Biochem,1993,215(1):113-122.
[9] Haussinger D, Lang F, Gerok W. Regulation of cell function by the celluar hydration state[J]. Am J Physiol,1994,267(3):E343-355.
收稿日期:1998-12-05;修回日期:1999-09-21, 百拇医药