神经生长因子(NGF)对H2O2诱导胶质瘤A-172细胞凋亡的抑制效应及其机制
作者:李忌 陈俊杰 王若菡
单位:李忌 陈俊杰 王若菡(华西医科大学基础医学院医学分子生物学研究室,四川成都610041)
关键词:神经生长因子;过氧化氢;胶质瘤A-172细胞;细胞凋亡;谷胱甘肽
癌症000503
【摘要】目的:研究神经生长因子(NGF)对过氧化氢(H2O2)诱导神经胶质瘤细胞凋亡的抑制作用及其机制。方法:抑制细胞增殖的检测采用MTT法,吖啶橙(AO)染色荧光显微观察细胞的形态学变化,DNA琼脂糖电泳检测DNA断裂,二硫硝基苯甲酸(DTNB)法检测细胞内还原型谷胱甘肽(GSH)水平的变化。结果:100~800μmol/L的H2O2明显抑制A-172细胞增殖,200μmol/LH2O2作用12h后,形态学上表现为染色体聚集、核固缩和断裂,电泳可见DNA断裂形成的阶梯状条带。经NGF作用24h后可引起A-172细胞内GSH水平上升近2倍,但NGF抑制H2O2诱导A-172细胞凋亡的效应与GSH水平无关,而且此过程不需新蛋白或RNA的合成。结论:H2O2可有效诱导神经胶质瘤A-172细胞凋亡,NGF对此表现显著抑制效应,但与细胞内GSH水平变化无关。
, 百拇医药
中图分类号:R739.63;R730.1文献标识码:A文章编号:1000-467X(2000)05-0404-05
Prevention and mechanism of H2O2-induced apoptosis by nerve growth factor in glioma cell line A-172
LI Ji, CHEN Jun-jie, WANG Ruo-han
(Medical Molecular Biology Laboratory, College of Basic Medical Sciences,West China University of Medical Sciences, Chengdu 610041, P.R. China)
【Abstract】 Objective: To investigate the protective effects of nerve growth factor (NGF) on hydrogen peroxide (H2O2)-induced apoptosis of glioma cell line A-172 and its mechanism. Methods: Inhibition of proliferation was measured with a colorimetric 3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide (MTT) assay. Morphological assessment of apoptosis was performed with acridine orange(AO) stained fluorescence microscope. DNA fragmentation was assessed by agarose gel electrophoresis. The level of intracellular glutathione (GSH) was measured with a colorimetric 5, 5'-dithio-bis(2-nitrobenzoic acid)(DTNB) assay. Results: Exposure of exponentially growing A-172 cells to H2O2 100~ 800 μ mol/L for 12 h resulted in growth arrest. After treatment of A-172 cells with H2O2 200 μmol/L for 12h, marked morphological changes including “ apo bodies” , chromatin condensed and fragmentation were observed with AO stained microscope. Agarose gel electrophoresis of DNA from cells treated with 200 μ mol/L H2O2 for 12 h revealed “ ladder” pattern. Treatment with NGF for 24 h increased the level of cellular antioxidant glutathione (GSH) by ~ 2.0 fold. However, NGF protected cells against H2O2-stress even when cellular GSH was depleted by treatment with L-buthionine-(S, R)-sulfoximine (BSO). The GSH-independent protection effects of NGF did not require new protein or RNA synthesis. Conclusion: H2O2 induces apoptosis of A-172 cells, and that NGF prevent apoptosis independently of level of intracellular GSH.
, 百拇医药
Key words: Nerve growth factor; Hydrogen peroxide;Glioma cell line A-172; Apoptosis; Glutathione
Increased level of reactive oxygen species (ROS) in cells, referred to oxidative stress, is cytotoxic and has been postulated to be the cause of neuronal degenerative diseases[1, 2]. Against oxidative stress, cells possess several cellular defense systems including the antioxidant enzymes and antioxidants, such as glutathione (GSH), vitamin E or β -carotene. Nerve growth factor (NGF) rescues cells from injury by ROS[3] and is revealed to increase both the level of cellular GSH and the activity of the antioxidant enzymes in neuronal cells[4]. However, it is still unclear how ROS induces cell death and how NGF protects cells from oxidative stress. Recently, we have been reported that H2O2 induced apoptosis[5]. Here we investigated whether H2O2 induces apoptosis in glioma cell line A-172 and the possible role of NGF in the process.
, 百拇医药
1 MATERIALS AND METHODS
1.1 Reagents
Culture medium was purchased from Gibco Laboratories (Santa Clara, CA). Fetal bovine serum (FBS) was obtained from Si-Ji-Qing Biotechnology Co. (Hangzhou, China). Trypsin, RNase A, 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bro-mide (MTT), nerve growth factor (NGF) and L-buthi-onine-(S, R)-sulfoximine (BSO) were obtained from Sigma (St. Louis, MO). Actinomycin D (AcD) and cycloheximide (CHX) were purchased from Fluka (Buchs, Switzerland). All other reagents were of analytical reagent quality.
, 百拇医药
1.2 Cell culture and treatment
A-172 cell line was obtained from American Type Culture Collection (Rockville, Maryland) and maintained in RPMI 1 640 culture medium suppleme-nted with 10% fetal bovine serum (FBS), penicillin/streptomycin, and L-glutamine.The cells were regularly subcultured by trypsinization [0.25% (w/v) trypsin in Ca2+ and Mg2+ -free phosphate buffered saline, D-Hank's, pH 7.2]. The H2O2 was directly added to the wells in culture medium at final concentrations ranging from 100 μ mol/L to 800 μ mol/L. Viable cells were counted using the MTT assay as previously described[6]. Briefly cells were seeded in 96-well culture plates at 1 × 104 cells per well in 100 μ l of growth medium and incubated at 37℃ for 24 h. Then H2O2 was added to the culture and an 100 μ l fresh medium was added to each well and followed by another 12h incubation for a further.10 μ l MTT solution (5 mg/ml in PBS, filter sterilised) was added to each well and incubation for a further 4h at 37℃ . The formazan product was solubilised by the addition of 100 μ l DMSO. The optial density of each well was measured using Bio-Rad Model 3 550 plate reader at 595 nm with reference at 655 nm. Wells containing culture medium and MTT but no cells acted as blanks. Cell survival was expressed as an absorbance (A) percentage defined by : [A(drug-blank)÷ A(control-blank)× 100].
, 百拇医药
1.3 Fluorescence microscopy analysis[7]
Cultured cells on the slide were theated by addition of added one drop of acridine orange (AO) solution (10 μ g/ml in PBS), and immediately examined with Olympus microscope with fluorescence attachment. Green fluorescence was detected between 500 and 525 nm.
1.4 DNA fragmentation assay[8]
After cultivation for the indicated peroid, cells were harvested on ice using a cell scraper. Following centrifugation at 4℃ at 1 500g for 5 min, cell pelle-ts were incubated at 37℃ for 2 h in a lysis buffer containing 10 mmol/L Tris-HCl buffer, pH8.0, 10 mmol/L EDTA, 1% sodium dodecyl sulfate (SDS) and 20 μ g/ml DNase-free RNase. Then, 200 μ g/ml of proteinase K was added and the sample was further incubated at 37℃ overnight. The DNA solution was extracted with equal volumes of phenol, phenol: chloroform: isoamyl alcohol (25∶ 24∶ 1) and chloroform,respectively. The DNA was precipitated in 0.2 mol/L sodium chloride with two volumes of 100% ethanol- 20℃ overnight, and centrifugoted at 12 000g at 4℃ for 20 min. The pellets were washed twice with 70% ethanol, air dried and resuspended in a TE buffer containing 10 mmol/L Tris-HCl and 1 mmol EDTA, pH7.4. The DNA concentration was determined from absorbance at 260 nm. Ten micro-grams each of DNA samples was subjected to ele-ctrophoresis on 1.5% agarose gel containing 0.1~ 0.5 μ g/ml ethidium bromide and visualized under UV light. Electropho-resis was carried out in TE buffer (10 mmol/L Tris-HCL, 1 mmol/L EDTA at pH 8.0) for 10h at 20 V.
, 百拇医药
1.5 Assay of intracellular GSH
GSH was assessed with DTNB [ 5, 5'-dithio-bis(2-nitrobenzoic acid)] based on the method as described[9]. A-172 cells (5× 106/ml) were soni-cated for 30 seconds in 300 μ l 5% 5-sulfosalicylic acid and centrifuged for 10 min at 1 000g. The resultant acid thiol extract was assayed for nonprotein sulfhydryls by quantitating the reduction of DTNB through its conversion to 5-thiol-2-nitrobenzoic acid (TNB) at 412 nm by using a spectrophotometer[10]. Sample values were then calculated from a standard curve generated with known amounts of GSH and expressed as nmol/107 cells.
, 百拇医药
2 RESULTS
When A-172 cells were exposed to oxidative stress by incubation with 200 μ mol/L H2O2 for 12 h, typical morphology of the apoptotic A-172 nuclei was observed (Fig.1b). The nuclei appear slightly smaller than that of normal A-172 nuclei (Fig.1a). The predominant characteristics in the nuclei of H2O2 treated cells (Fig.1b), compared to normal A-172 nuclei (Fig.1a), was the fragmented state of their nuclei. Moreover, marked fragmentations of cellular DNA into sizes of multiples of 180 bp were induced as shown in Fig.2. However, the degree of DNA fragmentation was apparently reduced at the higher concentration of H2O2 (400 μ mol/L), and almost all the cells showed signs of necrotic cell death with focal rupture of cell membranes and surface blebbing (data not shown).
, 百拇医药
A MTT reduction assay (Fig.3) revealed that NGF (50 ng/ml) promoted the survival of cells exposed to H2O2 at concentrations of 100~ 800 μ mol/L whi-ch induced apoptosis. In fact, the DNA fragmentation caused by 200 μ mol/L H2O2 was apparently reduced by NGF (Fig.2).
Fig.1 Fluorescence microscopy of acridine orange staining
(a)control; (b)200 μ mol/L H2O2 treatment.
, 百拇医药
Magnification (× 300).
Fig.2 DNA fragmentation identificated as typical ladder
pattern of apoptosis on 1.5% agarose gel.
1: Mecular weight marker (Hind III & EcoRI digest of phage ( DNA); 2: control A- 172 glioma cells incubated for 12 h; 3: treated with 200 μ mol/L H2O2 for 12 h; 4: treated with 400 μ mol/L H2O2 for 12h; 5: treated with NGF (50 ng/ml) + H2O2 (200 μ mol/L) for 12 h. The gel was stained with ethidium bromide and examined under UV illumination.
, http://www.100md.com
Fig.3 A-172 cells (2× 104/100 μ l per well of 96-well plates) were incubated with NGF for 24 h, then H2O2 was added to culture. After 24 h, 0.5 mg/ml MTT was added. The generated dark crystals were dissolved by DMSO, and the absorbance at 595 nm, with the reference at 655 nm, was measured.
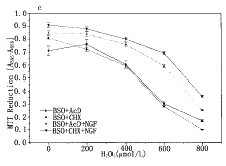
To clarify the mechanism of NGF to protect cells from oxidative stress, we estimated the level of cellular antioxidant GSH and the activities of catalase, GSH peroxidase, and GSH reductase. NGF did not increase the activities of these antioxidant enzymes within 24 h stimulation (data not shown), however, it was obvious that treatment with NGF for 24 h elevated cellular GSH level by ~ 2 fold (Fig.4a). To elucidate the relation between the GSH level and cell viability, the cellular GSH level was reduced by various concentrations of L-buthionine-(S, R)-sulfoximine (BSO), which is a specific inhibitor of γ -glutamylcycteine synthetase, the rate limiting enzyme of GSH synthesis (Fig.4a). Incubation with 100 μ mol/L H2O2 had no effect on cell viability in the absence of BSO, but more than 50% of cells died by the H2O2-treatment when cellular GSH was depleted by 100 μ mol/L BSO (Fig.4b). Howe-ver, it should be noted that NGF was capable of protecting cells against H2O2-stress, even when the cellular GSH was depleted (Fig.4b). These results suggest GSH-independent mechanism is involved in the protective effects of NGF against oxidative stress. With respect to the GSH-independent protection effects, NGF protected cell against H2O2-stress transcription and translation-independently (Fig.4c). As NGF also prevent serum deprivation-induced cell death[11], a common mecha-nism which does not require protein synthesis is suggested to be involved in the suppression of cell death by NGF.
, 百拇医药
3 DISCUSSION
ROS production plays an important role in physiologically-occuring cell death[12]. While supe-roxide (O2.- ) is the primary product of an e-attack on O2, it is a rather poorly reactive radical and it is not lipid soluble. Superoxide itself does not result in lipid peroxidation, a common component of cell damage. Most O2.- is converted to hydrogen peroxide (H2O2) and most damage is supposed to be mediated by the most reactive hydroxyl radical (OH), which is mainly generated from hydrogen peroxide through metal-catalyzed reaction: Fenton reaction.

, http://www.100md.com
Fig.4 Dose-dependent effect of BSO on cellular GSH
levels and on cell viability under H2O2-stress
(a) A-172 cells (1× 106/4 ml per 60-mm dish) were treated with various concentrations of BSO together with NGF for 24 h, then the GSH levels were determined as described in Materials and Methods. (b) A-172 cells (2× 104/100 μ l per well of 96-well plates) were treated with various concentrations of BSO in the prescence or absence of NGF for 24 h. Then H2O2 (200 μ mol/L) was added to the medium and further incubated for 24 h. (c) Actinomycin D (0.1 μ g/ml) or cycloheximide (1 μ g/ml) together BSO (100 μ mol/L) were added to A-172 cell culture (2× 104 cells/100 μ l per well of 96-well plates) 2 h before the addition of NGF (50 ng/ml). Twelve hours after, various concentrations of H2O2 were added and incubated further for 12 h. The data represented as means± s in triplicate experiments.
, 百拇医药
In this study, we demonstrated that exposure glioma cell line A-172 to the hydroxyl radical producer, hydrogen peroxide, can trigger cell death via apoptosis. Hydrogen peroxide-induced cell death is accompanied by the morphological and biochemical features of apoptosis. We surmised which target is the most important. One role may be as a signaling molcule that regulates the redox state and affects the activity of several transcription factors[14] and another may be lipid membrane peroxidation[15].
, 百拇医药
NGF reportedly protects PC12 cells from oxidative stress-induced apoptosis[3]. In this study, NGF parti-ally blocked H2O2-induced apoptosis. Several invsti-gators suggested that NGF reduce the intrace-llular level of ROS[13], Hockenbery et al[15] reported that NGF withdrawal induces ROS production associ-ated with apoptosis of synpathetic neurons. The data suggest that the survival effect of NGF as to the hydrogen peroxide-induced apoptosis is due to the reduced generation of ROS. The present results showed that NGF could increase the level of intracellular antioxidant GSH, but NGF was still capable of protecting cells against apoptosis of H2O2-stress even when the cellular GSH was depleted by BSO. The results imply that GSH-independent mechanism is involved in the protective effects of NGF against oxidative stress.
, http://www.100md.com
In conclusion, our data demonstrate that H2O2 triggers apoptosis of A-172 cells, and that NGF prevents apoptosis independently of level of intrace-llular GSH.
基金项目:本课题受中国博士后科学基金资助
[REFERENCES]
1,Fahn S, Cohen G. The oxidant stress hypothesis in Parkinson's disease evidence supporting it [J]. Ann Neurol, 1992, 32: 804~ 812.
2,Wiedau-Pazos M, Goto JJ, Rabizadeh S, et al. Altered reactivity of superoxide dismutase in familial amyotrophic lateral sclerosis [J]. Science, 1996, 271: 515~ 518.
, 百拇医药
3,Enokido Y, Hatanaka H. High oxygen atmosphre for neuronal cell culture with nerve growth factor. II. Survival and growth of clonal rat pheochromocytoma PC12 cells [J]. Brain Res, 1990, 536: 23~ 29.
4,Pan Z, Perez-Polo R. Role of nerve growth factor in oxidant homeostasis: glutathione metabolism [J]. J Neurochem, 1993, 61: 1713~ 1721.
5,Li J, Huang CY, Zheng RL, et al. Hydrogen peroxide induces apoptosis of human hepatoma cell and alters the cell redox status [J]. Cell Biol Int (in press) 2000.
, http://www.100md.com
6,Li J, Zheng Y, Zheng RL, et al. Antitumour effects of phenylpropanoid glycosides [J]. Chinese Pharmac J, 1995, 30: 269~ 271.
7,Shiff SJ, Koutsos MI, Qiao L, et al. Nonsteroidal antiinflammatory drugs inhibit the proliferation of colon adenocarcinoma cells: effects on cell cycle and apoptosis [J]. Exp Cell Res, 1996, 222: 179~ 188.
8,Chen J, Jin K, Chen M, et al. Early detection of DNA strand breaks in the brain after transient focal ischemia: implications for the role of DNA damage in apoptosis and neuronal cell death [J]. J Neurochem, 1997, 69: 232~ 245.
, 百拇医药
9,Jocelyn PC. Spectrophototemetric assay of thiols [J]. Methods EnzyM, 1987, 143: 44~ 67.
10,Watson RW, Rotstein OD, Nathens AB, et al. Thiol-mediated redox regulation of neutrophil apoptosis [J]. Surgery, 1996, 120: 150~ 158.
11,Rukenstein A, Rydel RE, Greene LA. Multiple agents rescue PC12 cells from serum-free cell death by translation-and transcription-independent mechanisms [J]. J Neurosci, 1991, 11: 2552~ 2563.
, 百拇医药
12,Greenlund LJ, Deckwerth TL, Johnson EM Jr. Superoxide dismutase delays neuronal apoptosis: A role for reactive oxygen species in programmed neuronal death [J]. Neuron, 1995, 14: 303~ 315.
13,Satoh T, Sakai N, Enokido Y, et al. Free radical-independent protection by nerve growth factor and Bcl-2 of PC12 cells from hydrogen peroxide-triggered apoptosis [J]. J Biochem, 1996, 120: 540~ 546.
14,Abate C, Patel L, Rauscher FJ 3d, et al. Redox regulation of fas and jun DNA-binding activity in vitro [J]. Science, 1990, 249: 1157~ 1161.
15,Hockenbery DM, Oltvai ZN, Yin XM, et al. Bcl-2 functions in an antioxidant pathway to prevent apoptosis [J]. Cell, 1993, 75: 241~ 251.
收稿日期:1999-11-09;修回日期:1999-12-23, 百拇医药
单位:李忌 陈俊杰 王若菡(华西医科大学基础医学院医学分子生物学研究室,四川成都610041)
关键词:神经生长因子;过氧化氢;胶质瘤A-172细胞;细胞凋亡;谷胱甘肽
癌症000503
【摘要】目的:研究神经生长因子(NGF)对过氧化氢(H2O2)诱导神经胶质瘤细胞凋亡的抑制作用及其机制。方法:抑制细胞增殖的检测采用MTT法,吖啶橙(AO)染色荧光显微观察细胞的形态学变化,DNA琼脂糖电泳检测DNA断裂,二硫硝基苯甲酸(DTNB)法检测细胞内还原型谷胱甘肽(GSH)水平的变化。结果:100~800μmol/L的H2O2明显抑制A-172细胞增殖,200μmol/LH2O2作用12h后,形态学上表现为染色体聚集、核固缩和断裂,电泳可见DNA断裂形成的阶梯状条带。经NGF作用24h后可引起A-172细胞内GSH水平上升近2倍,但NGF抑制H2O2诱导A-172细胞凋亡的效应与GSH水平无关,而且此过程不需新蛋白或RNA的合成。结论:H2O2可有效诱导神经胶质瘤A-172细胞凋亡,NGF对此表现显著抑制效应,但与细胞内GSH水平变化无关。
, 百拇医药
中图分类号:R739.63;R730.1文献标识码:A文章编号:1000-467X(2000)05-0404-05
Prevention and mechanism of H2O2-induced apoptosis by nerve growth factor in glioma cell line A-172
LI Ji, CHEN Jun-jie, WANG Ruo-han
(Medical Molecular Biology Laboratory, College of Basic Medical Sciences,West China University of Medical Sciences, Chengdu 610041, P.R. China)
【Abstract】 Objective: To investigate the protective effects of nerve growth factor (NGF) on hydrogen peroxide (H2O2)-induced apoptosis of glioma cell line A-172 and its mechanism. Methods: Inhibition of proliferation was measured with a colorimetric 3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide (MTT) assay. Morphological assessment of apoptosis was performed with acridine orange(AO) stained fluorescence microscope. DNA fragmentation was assessed by agarose gel electrophoresis. The level of intracellular glutathione (GSH) was measured with a colorimetric 5, 5'-dithio-bis(2-nitrobenzoic acid)(DTNB) assay. Results: Exposure of exponentially growing A-172 cells to H2O2 100~ 800 μ mol/L for 12 h resulted in growth arrest. After treatment of A-172 cells with H2O2 200 μmol/L for 12h, marked morphological changes including “ apo bodies” , chromatin condensed and fragmentation were observed with AO stained microscope. Agarose gel electrophoresis of DNA from cells treated with 200 μ mol/L H2O2 for 12 h revealed “ ladder” pattern. Treatment with NGF for 24 h increased the level of cellular antioxidant glutathione (GSH) by ~ 2.0 fold. However, NGF protected cells against H2O2-stress even when cellular GSH was depleted by treatment with L-buthionine-(S, R)-sulfoximine (BSO). The GSH-independent protection effects of NGF did not require new protein or RNA synthesis. Conclusion: H2O2 induces apoptosis of A-172 cells, and that NGF prevent apoptosis independently of level of intracellular GSH.
, 百拇医药
Key words: Nerve growth factor; Hydrogen peroxide;Glioma cell line A-172; Apoptosis; Glutathione
Increased level of reactive oxygen species (ROS) in cells, referred to oxidative stress, is cytotoxic and has been postulated to be the cause of neuronal degenerative diseases[1, 2]. Against oxidative stress, cells possess several cellular defense systems including the antioxidant enzymes and antioxidants, such as glutathione (GSH), vitamin E or β -carotene. Nerve growth factor (NGF) rescues cells from injury by ROS[3] and is revealed to increase both the level of cellular GSH and the activity of the antioxidant enzymes in neuronal cells[4]. However, it is still unclear how ROS induces cell death and how NGF protects cells from oxidative stress. Recently, we have been reported that H2O2 induced apoptosis[5]. Here we investigated whether H2O2 induces apoptosis in glioma cell line A-172 and the possible role of NGF in the process.
, 百拇医药
1 MATERIALS AND METHODS
1.1 Reagents
Culture medium was purchased from Gibco Laboratories (Santa Clara, CA). Fetal bovine serum (FBS) was obtained from Si-Ji-Qing Biotechnology Co. (Hangzhou, China). Trypsin, RNase A, 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bro-mide (MTT), nerve growth factor (NGF) and L-buthi-onine-(S, R)-sulfoximine (BSO) were obtained from Sigma (St. Louis, MO). Actinomycin D (AcD) and cycloheximide (CHX) were purchased from Fluka (Buchs, Switzerland). All other reagents were of analytical reagent quality.
, 百拇医药
1.2 Cell culture and treatment
A-172 cell line was obtained from American Type Culture Collection (Rockville, Maryland) and maintained in RPMI 1 640 culture medium suppleme-nted with 10% fetal bovine serum (FBS), penicillin/streptomycin, and L-glutamine.The cells were regularly subcultured by trypsinization [0.25% (w/v) trypsin in Ca2+ and Mg2+ -free phosphate buffered saline, D-Hank's, pH 7.2]. The H2O2 was directly added to the wells in culture medium at final concentrations ranging from 100 μ mol/L to 800 μ mol/L. Viable cells were counted using the MTT assay as previously described[6]. Briefly cells were seeded in 96-well culture plates at 1 × 104 cells per well in 100 μ l of growth medium and incubated at 37℃ for 24 h. Then H2O2 was added to the culture and an 100 μ l fresh medium was added to each well and followed by another 12h incubation for a further.10 μ l MTT solution (5 mg/ml in PBS, filter sterilised) was added to each well and incubation for a further 4h at 37℃ . The formazan product was solubilised by the addition of 100 μ l DMSO. The optial density of each well was measured using Bio-Rad Model 3 550 plate reader at 595 nm with reference at 655 nm. Wells containing culture medium and MTT but no cells acted as blanks. Cell survival was expressed as an absorbance (A) percentage defined by : [A(drug-blank)÷ A(control-blank)× 100].
, 百拇医药
1.3 Fluorescence microscopy analysis[7]
Cultured cells on the slide were theated by addition of added one drop of acridine orange (AO) solution (10 μ g/ml in PBS), and immediately examined with Olympus microscope with fluorescence attachment. Green fluorescence was detected between 500 and 525 nm.
1.4 DNA fragmentation assay[8]
After cultivation for the indicated peroid, cells were harvested on ice using a cell scraper. Following centrifugation at 4℃ at 1 500g for 5 min, cell pelle-ts were incubated at 37℃ for 2 h in a lysis buffer containing 10 mmol/L Tris-HCl buffer, pH8.0, 10 mmol/L EDTA, 1% sodium dodecyl sulfate (SDS) and 20 μ g/ml DNase-free RNase. Then, 200 μ g/ml of proteinase K was added and the sample was further incubated at 37℃ overnight. The DNA solution was extracted with equal volumes of phenol, phenol: chloroform: isoamyl alcohol (25∶ 24∶ 1) and chloroform,respectively. The DNA was precipitated in 0.2 mol/L sodium chloride with two volumes of 100% ethanol- 20℃ overnight, and centrifugoted at 12 000g at 4℃ for 20 min. The pellets were washed twice with 70% ethanol, air dried and resuspended in a TE buffer containing 10 mmol/L Tris-HCl and 1 mmol EDTA, pH7.4. The DNA concentration was determined from absorbance at 260 nm. Ten micro-grams each of DNA samples was subjected to ele-ctrophoresis on 1.5% agarose gel containing 0.1~ 0.5 μ g/ml ethidium bromide and visualized under UV light. Electropho-resis was carried out in TE buffer (10 mmol/L Tris-HCL, 1 mmol/L EDTA at pH 8.0) for 10h at 20 V.
, 百拇医药
1.5 Assay of intracellular GSH
GSH was assessed with DTNB [ 5, 5'-dithio-bis(2-nitrobenzoic acid)] based on the method as described[9]. A-172 cells (5× 106/ml) were soni-cated for 30 seconds in 300 μ l 5% 5-sulfosalicylic acid and centrifuged for 10 min at 1 000g. The resultant acid thiol extract was assayed for nonprotein sulfhydryls by quantitating the reduction of DTNB through its conversion to 5-thiol-2-nitrobenzoic acid (TNB) at 412 nm by using a spectrophotometer[10]. Sample values were then calculated from a standard curve generated with known amounts of GSH and expressed as nmol/107 cells.
, 百拇医药
2 RESULTS
When A-172 cells were exposed to oxidative stress by incubation with 200 μ mol/L H2O2 for 12 h, typical morphology of the apoptotic A-172 nuclei was observed (Fig.1b). The nuclei appear slightly smaller than that of normal A-172 nuclei (Fig.1a). The predominant characteristics in the nuclei of H2O2 treated cells (Fig.1b), compared to normal A-172 nuclei (Fig.1a), was the fragmented state of their nuclei. Moreover, marked fragmentations of cellular DNA into sizes of multiples of 180 bp were induced as shown in Fig.2. However, the degree of DNA fragmentation was apparently reduced at the higher concentration of H2O2 (400 μ mol/L), and almost all the cells showed signs of necrotic cell death with focal rupture of cell membranes and surface blebbing (data not shown).
, 百拇医药
A MTT reduction assay (Fig.3) revealed that NGF (50 ng/ml) promoted the survival of cells exposed to H2O2 at concentrations of 100~ 800 μ mol/L whi-ch induced apoptosis. In fact, the DNA fragmentation caused by 200 μ mol/L H2O2 was apparently reduced by NGF (Fig.2).

Fig.1 Fluorescence microscopy of acridine orange staining
(a)control; (b)200 μ mol/L H2O2 treatment.
, 百拇医药
Magnification (× 300).

Fig.2 DNA fragmentation identificated as typical ladder
pattern of apoptosis on 1.5% agarose gel.
1: Mecular weight marker (Hind III & EcoRI digest of phage ( DNA); 2: control A- 172 glioma cells incubated for 12 h; 3: treated with 200 μ mol/L H2O2 for 12 h; 4: treated with 400 μ mol/L H2O2 for 12h; 5: treated with NGF (50 ng/ml) + H2O2 (200 μ mol/L) for 12 h. The gel was stained with ethidium bromide and examined under UV illumination.

, http://www.100md.com
Fig.3 A-172 cells (2× 104/100 μ l per well of 96-well plates) were incubated with NGF for 24 h, then H2O2 was added to culture. After 24 h, 0.5 mg/ml MTT was added. The generated dark crystals were dissolved by DMSO, and the absorbance at 595 nm, with the reference at 655 nm, was measured.
To clarify the mechanism of NGF to protect cells from oxidative stress, we estimated the level of cellular antioxidant GSH and the activities of catalase, GSH peroxidase, and GSH reductase. NGF did not increase the activities of these antioxidant enzymes within 24 h stimulation (data not shown), however, it was obvious that treatment with NGF for 24 h elevated cellular GSH level by ~ 2 fold (Fig.4a). To elucidate the relation between the GSH level and cell viability, the cellular GSH level was reduced by various concentrations of L-buthionine-(S, R)-sulfoximine (BSO), which is a specific inhibitor of γ -glutamylcycteine synthetase, the rate limiting enzyme of GSH synthesis (Fig.4a). Incubation with 100 μ mol/L H2O2 had no effect on cell viability in the absence of BSO, but more than 50% of cells died by the H2O2-treatment when cellular GSH was depleted by 100 μ mol/L BSO (Fig.4b). Howe-ver, it should be noted that NGF was capable of protecting cells against H2O2-stress, even when the cellular GSH was depleted (Fig.4b). These results suggest GSH-independent mechanism is involved in the protective effects of NGF against oxidative stress. With respect to the GSH-independent protection effects, NGF protected cell against H2O2-stress transcription and translation-independently (Fig.4c). As NGF also prevent serum deprivation-induced cell death[11], a common mecha-nism which does not require protein synthesis is suggested to be involved in the suppression of cell death by NGF.
, 百拇医药
3 DISCUSSION
ROS production plays an important role in physiologically-occuring cell death[12]. While supe-roxide (O2.- ) is the primary product of an e-attack on O2, it is a rather poorly reactive radical and it is not lipid soluble. Superoxide itself does not result in lipid peroxidation, a common component of cell damage. Most O2.- is converted to hydrogen peroxide (H2O2) and most damage is supposed to be mediated by the most reactive hydroxyl radical (OH), which is mainly generated from hydrogen peroxide through metal-catalyzed reaction: Fenton reaction.



, http://www.100md.com
Fig.4 Dose-dependent effect of BSO on cellular GSH
levels and on cell viability under H2O2-stress
(a) A-172 cells (1× 106/4 ml per 60-mm dish) were treated with various concentrations of BSO together with NGF for 24 h, then the GSH levels were determined as described in Materials and Methods. (b) A-172 cells (2× 104/100 μ l per well of 96-well plates) were treated with various concentrations of BSO in the prescence or absence of NGF for 24 h. Then H2O2 (200 μ mol/L) was added to the medium and further incubated for 24 h. (c) Actinomycin D (0.1 μ g/ml) or cycloheximide (1 μ g/ml) together BSO (100 μ mol/L) were added to A-172 cell culture (2× 104 cells/100 μ l per well of 96-well plates) 2 h before the addition of NGF (50 ng/ml). Twelve hours after, various concentrations of H2O2 were added and incubated further for 12 h. The data represented as means± s in triplicate experiments.
, 百拇医药
In this study, we demonstrated that exposure glioma cell line A-172 to the hydroxyl radical producer, hydrogen peroxide, can trigger cell death via apoptosis. Hydrogen peroxide-induced cell death is accompanied by the morphological and biochemical features of apoptosis. We surmised which target is the most important. One role may be as a signaling molcule that regulates the redox state and affects the activity of several transcription factors[14] and another may be lipid membrane peroxidation[15].
, 百拇医药
NGF reportedly protects PC12 cells from oxidative stress-induced apoptosis[3]. In this study, NGF parti-ally blocked H2O2-induced apoptosis. Several invsti-gators suggested that NGF reduce the intrace-llular level of ROS[13], Hockenbery et al[15] reported that NGF withdrawal induces ROS production associ-ated with apoptosis of synpathetic neurons. The data suggest that the survival effect of NGF as to the hydrogen peroxide-induced apoptosis is due to the reduced generation of ROS. The present results showed that NGF could increase the level of intracellular antioxidant GSH, but NGF was still capable of protecting cells against apoptosis of H2O2-stress even when the cellular GSH was depleted by BSO. The results imply that GSH-independent mechanism is involved in the protective effects of NGF against oxidative stress.
, http://www.100md.com
In conclusion, our data demonstrate that H2O2 triggers apoptosis of A-172 cells, and that NGF prevents apoptosis independently of level of intrace-llular GSH.
基金项目:本课题受中国博士后科学基金资助
[REFERENCES]
1,Fahn S, Cohen G. The oxidant stress hypothesis in Parkinson's disease evidence supporting it [J]. Ann Neurol, 1992, 32: 804~ 812.
2,Wiedau-Pazos M, Goto JJ, Rabizadeh S, et al. Altered reactivity of superoxide dismutase in familial amyotrophic lateral sclerosis [J]. Science, 1996, 271: 515~ 518.
, 百拇医药
3,Enokido Y, Hatanaka H. High oxygen atmosphre for neuronal cell culture with nerve growth factor. II. Survival and growth of clonal rat pheochromocytoma PC12 cells [J]. Brain Res, 1990, 536: 23~ 29.
4,Pan Z, Perez-Polo R. Role of nerve growth factor in oxidant homeostasis: glutathione metabolism [J]. J Neurochem, 1993, 61: 1713~ 1721.
5,Li J, Huang CY, Zheng RL, et al. Hydrogen peroxide induces apoptosis of human hepatoma cell and alters the cell redox status [J]. Cell Biol Int (in press) 2000.
, http://www.100md.com
6,Li J, Zheng Y, Zheng RL, et al. Antitumour effects of phenylpropanoid glycosides [J]. Chinese Pharmac J, 1995, 30: 269~ 271.
7,Shiff SJ, Koutsos MI, Qiao L, et al. Nonsteroidal antiinflammatory drugs inhibit the proliferation of colon adenocarcinoma cells: effects on cell cycle and apoptosis [J]. Exp Cell Res, 1996, 222: 179~ 188.
8,Chen J, Jin K, Chen M, et al. Early detection of DNA strand breaks in the brain after transient focal ischemia: implications for the role of DNA damage in apoptosis and neuronal cell death [J]. J Neurochem, 1997, 69: 232~ 245.
, 百拇医药
9,Jocelyn PC. Spectrophototemetric assay of thiols [J]. Methods EnzyM, 1987, 143: 44~ 67.
10,Watson RW, Rotstein OD, Nathens AB, et al. Thiol-mediated redox regulation of neutrophil apoptosis [J]. Surgery, 1996, 120: 150~ 158.
11,Rukenstein A, Rydel RE, Greene LA. Multiple agents rescue PC12 cells from serum-free cell death by translation-and transcription-independent mechanisms [J]. J Neurosci, 1991, 11: 2552~ 2563.
, 百拇医药
12,Greenlund LJ, Deckwerth TL, Johnson EM Jr. Superoxide dismutase delays neuronal apoptosis: A role for reactive oxygen species in programmed neuronal death [J]. Neuron, 1995, 14: 303~ 315.
13,Satoh T, Sakai N, Enokido Y, et al. Free radical-independent protection by nerve growth factor and Bcl-2 of PC12 cells from hydrogen peroxide-triggered apoptosis [J]. J Biochem, 1996, 120: 540~ 546.
14,Abate C, Patel L, Rauscher FJ 3d, et al. Redox regulation of fas and jun DNA-binding activity in vitro [J]. Science, 1990, 249: 1157~ 1161.
15,Hockenbery DM, Oltvai ZN, Yin XM, et al. Bcl-2 functions in an antioxidant pathway to prevent apoptosis [J]. Cell, 1993, 75: 241~ 251.
收稿日期:1999-11-09;修回日期:1999-12-23, 百拇医药