猫脊髓后角浅层内GABA能神经终末与表达阿片μ受体神经元之间的突触联系*
作者:龚良维 丁玉强 郑恒兴 王 丹 秦秉志 李继硕
单位:第四军医大学解剖学教研室,梁銶琚脑研究中心,西安 710032
关键词:阿片μ受体;GABA电镜;免疫组织化学;脊髓;猫
解剖学报980301 摘 要 为了研究GABA与MOR在吗啡镇痛中的相互关系,用包埋前免疫组织化学方法结
合包埋后胶体金电镜技术,观察了猫脊髓后角浅层内γ-氨基丁酸(GABA)能轴突终末与表达阿片μ受体神经元之间的突触联系。结果表明,后角浅层内约有三分之一的MOR阳性树突和胞体与GABA阳性终末形成突触连接,这些突触均为对称性突触。本实验结果提示猫脊髓后角浅层内MOR阳性神经元的功能活动可能受到突触前成分释放的GABA的影响。
γ-氨基丁酸(GABA)是哺乳动物中枢神经系统内的一种抑制性神经递质[1]。脊髓后角浅层内存在大量的GABA能终末[2],其主要来源为本节段和其他节段的GABA能中间神经元的终末、脑干的GABA能神经元的下行投射纤维及外周初级传入纤维。以往的研究表明GABA在脊髓水平对伤害性刺激具有抑制作用[3]。如离子微电泳或静脉注射GABA可选择性地抑制伤害性刺激引起的后角神经元的反应,脊髓蛛网膜下腔注射GABAB受体激动剂苯氯丁氨酸(baclofen)可产生长时程的镇痛效应[4]。
, http://www.100md.com
阿片受体至少包括3种亚型:μ、δ和κ。其中μ受体(mu-opioid receptor;MOR)和吗啡的镇痛作用密切相关[5,6]。放射自显影[7]、原位杂交[8]及免疫组织化学[9]均证实脊髓后角浅层分布有大量MOR。在内源性的阿片类物质中,甲硫氨酸脑啡肽(M-ENK)与克隆的MOR亲和力最强[10]。M-ENK在脊髓内主要分布于后角浅层,且部分M-ENK阳性神经元同时含有GABA[11],提示后角浅层内GABA能终末可能与MOR阳性神经元之间存在直接的突触联系。因此,本实验在对猫脊髓后角浅层内MOR免疫阳性成分电镜观察的基础上[12],对MOR阳性成分与GABA能终末之间的突触联系进行了探讨,旨在为研究GABA与MOR在吗啡镇痛中相互关系提供线索。
材料和方法
实验共用成年雄猫4只。动物在腹腔注射戊巴比妥钠的深麻状态下,先用500ml生理盐水经心脏冲洗血液,然后用1 000ml含4%多聚甲醛,1%苦味酸和0.5%戊二醛的磷酸缓冲液(0.1mol/L PB,pH7.4)灌注固定。立即取出腰骶段脊髓,并在上述固定液内后固定3h(4℃),振动切片机切片,厚50μm,切片收集于磷酸盐缓冲液(0.01mol/L PBS,pH7.4)内。按ABC法进行免疫组织化学染色。切片在经PBS漂洗后依次移入:(1)豚鼠抗大鼠MOR的IgG(0.5mg/L)[9],室温孵育过夜;(2)结合有生物素的羊抗豚鼠IgG(Vector;1∶200),室温孵育3h;(3)ABC复合物(Vector;1∶200),室温孵育2h。其中,一抗和二抗用含0.01% Triton X-100,0.2%驴血清和0.03%NaN3的PBS稀释,ABC复合物用含0.01%Triton X-100的PBS稀释。最后切片在含0.05%DAB和0.003%H2O2的0.05%mol/L Tris-HCl缓冲液内呈色20~30min。切片经漂洗后在含1%锇酸的PB内室温下固定1h,梯度酒精及环氧丙烷脱水,Epon812平板包埋、聚合(45℃,24h;58℃,48h)。在解剖镜下取出后角浅层,LKB超薄切片机切片并裱于镍网上,然后进行GABA免疫组织化学染色,将镍网置于含兔抗大鼠GABA血清(1∶1 000)和0.01%Triton X-100的Tris-HCl缓冲液(TBST;pH7.6)液滴上,室温下湿盒内孵育24h。经pH7.6的TBST清洗后,再用pH8.2的TBST清洗,然后置于含结合有胶体金(直径10nm)颗粒的羊抗兔IgG(1∶40, British BioCell International)的液滴上,室温下湿盒内孵育3h。醋酸铀,枸橼酸铅染色,Hitachi100电子显微镜观察,摄片。
, 百拇医药
对照实验在省略一抗或用正常豚鼠血清或兔血清代替一抗的情况下进行。
结 果
用正常豚鼠血清替代或省略抗MOR抗体的条件下进行的对照实验显示,DAB呈色后的切片上只见有浅淡的背底,电镜下未观察到免疫反应阳性产物。包埋后GABA胶体金染色的对照实验亦在同样的条件(省略一抗或用正常兔血清替代)下进行,仅见到个别散在分布的胶体金颗粒。
与我们[12]先前的研究结果一致,猫脊髓后角浅层内MOR样免疫反应阳性产物主要分布于树突内,仅有少量的胞体及轴突呈现MOR阳性。在胞体内,阳性产物主要位于胞膜下,在胞浆内则主要位于粗面内质网的核糖体上。在树突内,阳性产物不仅出现于突触后膜下,亦分布于非突触部位的胞膜下,但前者多于后者(图1~3)。当1个神经终末内金颗粒数量大于其周围同等面积的非轴突结构内金颗粒数量的10倍以上者,则判定为GABA阳性[13]。GABA阳性轴突终末内含有多形性突触小泡,形成的突触为对称性突触(图1~3)。
, 百拇医药
双标电镜观察发现有相当数量的GABA阳性轴突终末与MOR阳性树突或胞体形成突触连接,其中多为轴树突触,偶见轴体突触,这些突触均为对称性突触(图1~4)。在所计数的100个有明确突触关系的MOR阳性树突和胞体中,其中有32个与GABA阳性轴突终末形成突触连接。实验中未发现有MOR阳性轴突含有GABA,也未见有MOR阳性的胞体为GABA阳性。
讨 论
MOR与吗啡的镇痛密切相关。MOR是通过与阿片类物质结合而发挥其生理效应。本研究结果表明,猫脊髓后角浅层内有约三分之一的MOR阳性的树突和胞体与GABA阳性终末形成突触连接,且全部为对称性突触。说明脊髓后角浅层内MOR神经元可能接受GABA能神经终末的抑制。以往的研究表明,脊髓后角浅层内约有70%的M-ENK阳性神经元同时含有GABA,提示M-ENK与GABA可能共存于轴突终末内[11]。此外,后角浅层神经元的膜片钳工作发现,GABA引起的氯电流可被MOR特异性的激动剂DAMGO增强[14]。因此,在伤害性刺激存在的情况下,后角浅层内GABA阳性轴突终末内的GABA可能与M-ENK一起释放,M-ENK作为内源性的吗啡类物质激活MOR阳性神经元而产生镇痛效应,而GABA可进一步增强M-ENK的镇痛效应。
, 百拇医药
此外,值得指出的是,本实验发现约三分之一的MOR阳性树突或胞体与GABA阳性终末之间存在突触联系,提示MOR阳性神经元除与GABA能神经终末形成突触外,可能还与含有其他神经活性物质的轴突终末形成突触。后角浅层内分布有大量不同来源且含有多种神经活性物质的神经终末,如5-羟色胺,降钙素基因相关肽,血管活性肠多肽等。这些神经活性物质均与伤害性刺激信息的传递或调制有着密切关系。MOR阳性神经元与这些神经活性物质之间的突触联系值得进一步探讨。
收稿1997-1 修回1997-07
图版说明
图1 示MOR阳性树突(De)与1个GABA能轴突终末(Ax)形成突触连接。箭头示突触活性点。标尺示0.2μm
图2 示MOR阳性树突(De)同时与2个GABA能轴突终末(Ax、Ax′)形成突触连接。箭头示突触点。标尺示0.3μm
, 百拇医药
图3 示MOR阳性树突(De)同时与1个GABA能轴突终末(Ax)及1个GABA阴性轴突终末(Ax′)形成突触连接。箭头示突触活性点。标尺示0.3μm
图4 示MOR阳性胞体(S)与1个GABA能轴突终末(Ax)形成突触连接。箭头示突触活性点。标尺示0.3μm
Explanation of figures
Fig.1 Showing synaptic contact between a MOR-positive dendrite(De)and a GABAergic axonal terminal(Ax).Arrow points to synaptic site.Bar=0.2μm
Fig.2 Showing a MOR-positive dendrite(De)makes synaptic contact with two GABAergic axonal terminals(Ax,Ax′).Arrows point to synaptic sites.Bar=0.3μm
, 百拇医药
Fig.3 Showing a MOR-positive dendrite(De)makes synaptic contacts with a GABAergic axonal
terminal(Ax)and a non-GABAergic axonal terminal(Ax′).Arrows point to synaptic sites.Bar=0.3μm
Fig.4 Showing synaptic contact between an MOR-positive cell body(S)and a GABAergic axonal terminal(Ax).Arrow points to synaptic site.Bar=0.3μm
* 国家自然科学基金资助课题(No.39600045)
参考文献
, http://www.100md.com
[1]Roberts E.γ-Aminbutyric acid and nervous system function.A perspective.Biochem Pharmac,1974,23(4):2639
[2]Todd AJ,McKenzie J.GABA-immunoreactive neuron in the dorsal horn of the rat spinal cord.Neuroscience,1989,31(3):799
[3]Cutting DA,Jordan CC.Alternative approaches to analgesia:Baclofer as a model compound.Bri J Pharmacol,1975,54(1):171
[4]Sawynok J.GABAergic mechanisms of analgesia:An update.Pharmocol Biochem and Behavior,1987,26(2):463
, http://www.100md.com
[5]Besson J-M,Chaouch A.Peripheral and spinal mechanisms of nociception.Physiol Rev,1987,67(1):67
[6]Mansour A,Waston SJ,Akil H.Opioid receptors:Past,present and future.Trends Neurosci,1995,18(1):69
[7]Atweh SF,Kuhar MJ.Autoradiographic localization of opiate receptor in rat brain.I spinal cord and lower medulla.Brain Res,1977,124(1):53
[8]Minami M,Onogi T,Toya T,et al.Molecular cloning and in situ hybridization histochemistry for rat μ-opioid receptor.Neurosci Res,1994,18(2):315
, 百拇医药
[9]Ding YQ,Kaneko T,Momura S,et al.Immunohistochemical localization of μ-opioid receptors in the central nervous sytem of the rat.J Comp Neurol,1996,367(1):375
[10]Raynor K,Kong H,Chen Y,et al.Pharmacological characteriztion of the cloned κ-,δ-,and μ-opioid receptors.Mol Pharmacol,1994,45(2):330
[11]Todd AJ,Spike RC,Russell G,et al.Immunohistochemical evidence that Met-Enkephalin and GABA coexist in some neurons in rat dorsal horn.Brain Res,1992,584(1):149
, http://www.100md.com
[12]王 丹,龚良维,丁玉强等.阿片μ受体在猫脊髓背角浅层内的超微定位分布研究.解剖学报,1998,29(1):30
[13]Phend KD,Weinberg RJ,Rustioni A.Techniques to optimize post-embedding single and double staining for amino acid neurotransmitters.J Histochem Cytochem,1992,40(4):1011
[14]Wang RA,Rardic M.Activation of μ-opioid receptor modulates BABAA receptor-mediated currents in isolated spinal horn neurons.Neurosci Lett,1994,180(1):109
, 百拇医药
THE SYNAPTIC RELATIONSHIP BETWEEN GABAERGIC AXON
TERMINALS AND NEURONS EXPRESSING μ-OPIOID
RECEPTOR IN THE SUPERFICIAL DORSAL
HORN OF THE CAT SPINAL CORD
Gong Liangwei,Ding Yuqiang△,Zheng Hengxing,Wang Dan,Qin Bingzhi,Li Jishuo
(Department of Anatomy and K.K.Leung Brain Research Center,Fourth Military Medical University,Xi'an)
, 百拇医药
The synaptic relationship between GABAergic axon terminals and neurons expressing μ-opioid receptor(MOR)in the superficial spinal dorsal horn of the cat was examined by using pre-embedding immunohistochemical method combined with post-embedding immunogold technique.About one third of the cell bodies and dendrites showing MOR-like immunoreactivity formed synaptic contacts with GABAergic axon terminals;all these synapses were symmetric.The present results suggest MOR-positive neurons in the superficial layers of the cat spinal cord may be modulated by GABA releasing from presynaptic axon terminals.
, 百拇医药
KEY WORDS μ-Opioid receptor;GABA;Electron microscopy;Immunohistochemistry;Spinal cord;Cat
△Department of Anatomy and K.K.Leung Brain Research Center,Fourth Military Medical University,Xi'an 710032,China
ULTRASTRUCTURAL LOCALIZATION OF SUBSTANCE P
RECEPTOR AND ITS SYNAPTIC RELATIONSHIP WITH
THE PRIMARY AFFERENTS IN THE SUPERFICIAL
, 百拇医药
DORSAL HORN OF THE RAT SPINAL CORD
Tao Yuanxiang,Li Yunqing*,Zhao Zhiqi
(Shanghai Brain Research Institute,Chinese Academy of Sciences,Shanghai 200031,China;
* Department of Anatomy,The Fourth Military Medical University,Xi'an 710032,China)
Abstract The ultrastructural localization of substance P receptor within the superficial dorsal horn of rat spinal cord was observed in the present study.Besides in dendritic profiles and cell bodies,substance P receptor-like immunoreactive(SPR-LI)products could first be found in axonal profiles in the superficial dorsal horn.By a combined methods of transganglionic transport of horseradish peroxidase conjugated with wheat-germ agglutinin(WGA-HRP)and immunocytochemistry,it was observed that some SPR-LI dendritic profiles received synaptic contacts from,or were apposed directly to,WGA-HRP-labeled primary afferent terminals in the superficial laminae.
, 百拇医药
KEY WORDS Substance P receptor;Primary afferent;Ultrastructure;Dorsal horn;Spinal cord;Rat
Introduction
The undecapeptide substance P(SP) has been widely studied as mediator of sensory information in the nervous system.In the spinal cord,SP and SP receptor(SPR) are mainly present in the superficial laminae of spinal dorsal horn[1-4],at the sites of small-diameter primary afferent termination,suggesting that SP and SPR may be involved in spinal nociceptive mechanism.Although the ultrasturctural localization of SPR within the superficial laminae has been reported in the previous studies[5],the detailed observation of SPR ultrastructural distribution is lacking.Since it has been known that many small-diameter primary afferent fibers contain SP[1],the neurons with SPR in the superficial laminae probably receive nociceptive information from small-diameter primary afferent fibers.However,morphological evidence is lacking.The present study was attempted to observe the detailed distribution of SPR at the electron microscopic level and the relationship between SPR-like immunoreactive(SPR-LI) neurons and the small-diameter primary afferent fibers in the superficial laminae of spinal cord by the methods of immunocytochemical staining of SPR and transganglionic transport of horseradish peroxidase conjugated with wheat-germ agglutinin(WGA-HRP).
, 百拇医药
Materials and methods
The experiment was performed on 14 male Sprague-Dawley rats(230-280g).The distribution of SPR-LI neurons in the spinal dorsal horn was examined in 6 rats.The rats were anesthetized deeply with sodium pentobarbital (60mg/kg,i.p.)and perfused transcardially with 100ml of 0.01mol/L phosphate-buffered saline(PBS,pH 7.4),followed by 400ml of a fixative containing 4% paraformaldehyde,0.5% glutaradehyde and 0.1% picric acid in 0.1mol/L phosphate buffer(PB,pH 7.4).The lumbar segment was removed,and cut into 50μm thick transverse sections on a vibrotome.According to the avidin-biotinylated peroxidase complex method[6],the sections were incubated in rabbit anti-SPR antiserum(1mg/L)(gift from Prof.N Mizuno) diluted in PBS containing 3% normal goat serum for 48h at 4℃,and in biotinylated goat anti-rabbit IgG(1∶200 Vector)for 2h at 37℃ and in avidin-biotin-peroxidase complex(1∶100 Vector),for 1 h at 37℃.The immunoreactivity was visualized by incubation in the Tris-HCl buffer containing 0.05% DAB and 0.01% hydrogen peroxide for 5-8min.Some sections were mounted on glass slides,dehydrated,cleared and coverslipped with OPX for light microscopy.
, 百拇医药
For electron microscopy,the rest of the SPR-immunostained sections were postfixed with 1% osmium tetraoxide in 0.1mol/L PB for 45min at room temperature,and dehydrated in graduated ethanol,and finally embedded in Epon.The ultrathin sections were counterstained with urany1 acetate and lead citrate.The observation was made with Phillips CM-100 electron microscope.
A combination of transganglionic transport of WGA-HRP and SPR immunocytochemistry was performed in the remaining 8 rats.Following anesthesia with sodium pentobarbital (40mg/kg,i.p.),the sciatic nerve was exposed above the knee and injected underneath the epineurial sheath with 2% WGA-HRP(1-1.5μl,Sigma).
, 百拇医药
Three days later,the animals were perfused.The sections were prepared as described above,and processed according to a tungstate/tetramethylbenzidine(TMB) method to reveal peroxidase[7]and stabilized with a 3.3-diaminobenzidine(DAB)/cobalt/H2O2 solution.Some sections were directly mounted onto glass slides to examine the distribution of WGA-HRP-labeled terminals.Rest of the sections were processed for the SPR immunocytochemistry as described above.
, http://www.100md.com
In the control experiment,when the primary antibody was omitted or replaced with normal rabbit serum,no immunoreactivity was detectable.
Results
A dense population of SPR-LI neurons were seen in lamina I,particularly in its medial part(Fig.1).The most common type of SPR-LI neurons had a fusiform cell body and an extensive rostrocaudal dendritic tree,which was typically confined to lamina I and the outer portion of lamina Ⅱ,and occasionally reached the inner portion of laminae Ⅱ and Ⅲ.Contrasting sharply with lamina Ⅰ,lamina Ⅱ contained very few SPR-LI neurons(Fig.1).However,it contained a significant population of processes that derived from SPR-LI neurons located in lamina Ⅰ and the deeper laminae of the dorsal horn.A few SPR-LI neurons were sparsely distributed in laminae Ⅲ-Ⅵ.Some of these neuronal processes extended dorsally into the superficial laminae.
, 百拇医药
The terminals labeled by WGA-HRP were confined to the superficial dorsal horn.The labeled terminals were more intense in lamina Ⅱ than in lamina Ⅰ(Fig.2).No terminals labeled by WGA-HRP were found in the rest areas of the dorsal horn.
Electron microscopic observation was made on the ultrathin sections obtained from the superficial dorsal horn.Cytoplasmic SPR-LI products were in granular or patch associated with the microtubules,rough endoplasmic reticulum,Golgi bodies and surface of mitochondria.A small number of SPR-LI cell bodies,which contained a large indented nucleus,were often observed in lamina Ⅰ(Fig.3).SPR-LI products were most often encountered in the dendritic profiles(Fig.4).The reaction products distributed along the dendritic membrane were not in a continuous fashion,and not only in postsynaptic sites,but also in non-synaptic areas.Synaptic contacts onto these SPR-LI dendrites were symmetrical or asymmetrical.The presynaptic terminals often exhibited numerous round clear vesicles and /or a few dense core ones.The dense core vesicles were located near or away from the junctional membrane(Fig.4).A direct apposition between two SPR-LI dendrites was found(Fig.5).As shown in Fig.6,SPR-LI products were also seen in the axonal profiles,which contained mitochondria and numerous clear vesicles.SPR-LI axons and terminals occasionally formed synaptic contacts with dendrites profiles.No glial profiles were found to contain immunoreactive products.
, 百拇医药
The highest density of WGA-HRP-labeled terminals was often observed in lamina Ⅱ.TMB reaction product was generally in the form of electron-dense crystals.Most of the labeled terminals in the superficial dorsal horn formed symmetric or asymmetric synapse with dendrites.Some formed the central element of complex glomerular arrangements(Fig.7).It was observed,particularly in lamina Ⅱ,that some WGA-HPR-labeled terminals contacted synaptically with(Fig.8),or apposed directly to,the SPR-LI dendrites.Of 260 SPR-LI dendritic profiles,153 were directly apposed to the terminals 34 of which were WGA-HRP-labeled terminals,and 96 received synaptic contacts from terminals 16 of which were WGA-HRP-labeled terminals.The remaining 11 SPR-LI dendrites were in close proximity to the neuroglia,vessels and unstained cell bodies.
, 百拇医药
Discussion
In the spinal cord,the distribution pattern of SPR-like immunoreactivities in the present study was in agreement with that in the previous studies[2,3].SPR-LI neurons were located mainly in lamina Ⅰ and very few in lamina Ⅱ.However,lamina Ⅱ contained some processes from SPR-LI neurons located in lamina Ⅰ and the deep laminae of the dorsal horn.The present result further showed that SPR-LI dendrities in the superficial laminae received synaptic junction with or direct apposition to WGA-HRP-labeled primary afferent terminals.This datum strongly provided a morphological substrate for spinal nociception mediated by NK-1 receptor(substance P preferring binding receptor)[8-11].
, 百拇医药
An important finding in the present ultrastructural observation was the existence of SPR-LI axonal terminals within the superficial dorsal horn.Although the origin of such SPR axonal terminals was unclear,the following results may give some hints.(1) Andoh et al reported that there was the expression of NK-1 receptor mRNA in the mouse dorsal root ganglion(DRG)neuron[12];(2) Our recent study showed NK-1 receptor mRNA expressed in xenopus oocyte following an injection of total mRNA extracted from cat DRG[13];(3) Carlton et al has demonstrated that SPR was associated with unmyelinated axons of rat glabrous skin[14].Taken together,it is conceivable that SPR-LI axonal terminals in the present study might stem from the central axon of the DRG neurons.SPR,likely as an autoreceptor,may be involved in modulation of release of transmitters from primary afferent terminals[15].Howerver,since there was no change of SPR in the spinal cord following the cutting of the dorsal root or the destroy of unmyelinated sensory fibers by capsaicin[2],the other origins of SPR-LI axonal terminals could not be ruled out.
, 百拇医药
The authors wish to thank Professor Chang H T and Li J S for their helpful criticism and revision of the manuscript,and Professor Mizuno N for giving us rabbit anti-SPR antiserum,and are grateful to Ms.Zhang M L and Wang D for electron microscopic and pholographic help.This work was supported in part by the Grant from the NNSFC.
Received Juny 1997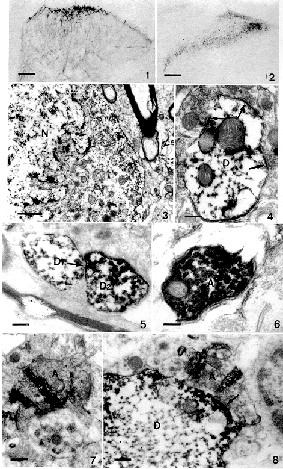
Explanation of figures
, 百拇医药
Fig.1 The distribution of SPR-positive neurons in the superficial dorsal horn.Scale bar:100μm
Fig.2 The distribution of WGA-HRP-labeled terminals in the superficial dorsal horn.Bar=100μm
Fig.3 The SPR-LI neuronal body with a indented nucleus(N)in lamina Ⅰ.Scale bar:1μm
Fig.4 SPR-LI dendrite(D)receiving synaptic contacts(arrows)from unstained terminals.Bar=0.25μm
, http://www.100md.com
Fig.5 A direct apposition(arrow)between two SPR-LI dendrites(D1,D2). bar=0.25μm
Fig.6 A SPR-LI axonal terminal(A).Bar=0.125μm
Fig.7 A WGA-HRP-labeled terminal (A) formed the central element of complex glomerulus arrangments in laminae Ⅱ.Bar=0.25μm
Fig.8 A synaptic contact(arrow)between a WGA-HRP-labeled terminal(A) and a SPR-LI dendrite(D).Bar=0.33μm
References
, http://www.100md.com
[1]Biasi S D,Rustioni A.Glutamate and substance P coexist in primary afferent terminals in the superficial laminae of spinal cord.Proc Nat1 Acad Sci U S A,1988,85(20):7820
[2]Brown J L,Liu H,Maggio J E,et al.,Morphological characterization of substance P receptor-immunoreactive neurons in the rat spinal cord and trigminal nucleus caudalis.J Comp Neurol,1995,356(3):327
[3]Nakaya Y,Kaneko,T.Shigemoto R,et al.Immunohistochemical localization of substance P receptor in the central nervous sytem of the adult rat.J Comp Neurol,1994,347(2):249
, 百拇医药
[4]Ruda M A.The pattern and place of nociceptive modulation in the dorsal horn.In:Yaksh T L(ed).Spinal Afferent Processing.New York,Plenum publishing corporation,1986,141-161
[5]Liu H,Brown J I,Jasmin L,et al.Synaptic relations between substance P and the substance P receptor:Light and electron microscopic characterization of the mismatch between neuropeptide and their receptor.Pro Nat1 Acad Sci USA,1994,91(3):1009
[6]Hsu S,Raine L,Fanger H A.A comparative study of the anti-peroxidase method and an avidinbiotin complex method for studying polypeptide hormones with radioimmunoassay antibodies.Am J Clin Pathol,1981,75(3):734
, 百拇医药
[7]Weinberg R J Van Eyck S L.A tertramethylbenzidine/tungstate reaction for horseradish peroxidase histochemistry.J Histochem Cytochem,1991,39(8):1143
[8]Murase K,Ryu P D,Randic M.Substance P augments a persistent slow inward calcium sensitive current in voltage-clamped rat spinal dorsal horn neurons of the rat.Brain Res,1986,365(2):369
[9]Murase K,Ryu P D,Randic M.Tachykinins modulate multiple ionic conductances in voltage-clamped rat spinal dorsal horn neurons.J Neurophysiol,1989,61(3):854
, http://www.100md.com
[10]Tao Y X,Wei F Zhao Z Q.A contribution of neurokinin-1 receptor to foramlin-induced c-fos expression in the rat spinal dorsal horn.Neurosci Lett,1997,221(2,3):105
[11]Wei F,Zhao Z Q.Blcokade of capsaicin-induced reduction of GABA-immunoreactivity by spantide in cat spinal superficial dorsal horn.Neuroscience,1996,71:(1):277
[12]Andoh T,Nagasawa T,Kuraishi Y.Expression of tachykinin NK1 receptor mRNA in dorsal root ganglia of the mouse.Mol Brain Res,1996,35(2):329
, 百拇医药
[13]Li H S,Bao Y D,Zhao Z Q.Expression of tachykinin receptor in Xenopus oocytes injected with poly(A)+RNA from cat dorsal root ganglion.Science in China Series C,1988,41(2):139
[14]Carlton S M,Zhou S,Coggeshall R E.Localization and activation of substance P receptor in unmyelinated axons of rat glabrous skin.Brain Res,1996,734(1):103
[15]Rusin K I,Ryu P D Randic M.Modulation of excitatory amino acid responses in rat dorsal horn neurons by tachykinins.J Neurophysiol,1992,68(2):265, 百拇医药
单位:第四军医大学解剖学教研室,梁銶琚脑研究中心,西安 710032
关键词:阿片μ受体;GABA电镜;免疫组织化学;脊髓;猫
解剖学报980301 摘 要 为了研究GABA与MOR在吗啡镇痛中的相互关系,用包埋前免疫组织化学方法结
合包埋后胶体金电镜技术,观察了猫脊髓后角浅层内γ-氨基丁酸(GABA)能轴突终末与表达阿片μ受体神经元之间的突触联系。结果表明,后角浅层内约有三分之一的MOR阳性树突和胞体与GABA阳性终末形成突触连接,这些突触均为对称性突触。本实验结果提示猫脊髓后角浅层内MOR阳性神经元的功能活动可能受到突触前成分释放的GABA的影响。
γ-氨基丁酸(GABA)是哺乳动物中枢神经系统内的一种抑制性神经递质[1]。脊髓后角浅层内存在大量的GABA能终末[2],其主要来源为本节段和其他节段的GABA能中间神经元的终末、脑干的GABA能神经元的下行投射纤维及外周初级传入纤维。以往的研究表明GABA在脊髓水平对伤害性刺激具有抑制作用[3]。如离子微电泳或静脉注射GABA可选择性地抑制伤害性刺激引起的后角神经元的反应,脊髓蛛网膜下腔注射GABAB受体激动剂苯氯丁氨酸(baclofen)可产生长时程的镇痛效应[4]。
, http://www.100md.com
阿片受体至少包括3种亚型:μ、δ和κ。其中μ受体(mu-opioid receptor;MOR)和吗啡的镇痛作用密切相关[5,6]。放射自显影[7]、原位杂交[8]及免疫组织化学[9]均证实脊髓后角浅层分布有大量MOR。在内源性的阿片类物质中,甲硫氨酸脑啡肽(M-ENK)与克隆的MOR亲和力最强[10]。M-ENK在脊髓内主要分布于后角浅层,且部分M-ENK阳性神经元同时含有GABA[11],提示后角浅层内GABA能终末可能与MOR阳性神经元之间存在直接的突触联系。因此,本实验在对猫脊髓后角浅层内MOR免疫阳性成分电镜观察的基础上[12],对MOR阳性成分与GABA能终末之间的突触联系进行了探讨,旨在为研究GABA与MOR在吗啡镇痛中相互关系提供线索。
材料和方法
实验共用成年雄猫4只。动物在腹腔注射戊巴比妥钠的深麻状态下,先用500ml生理盐水经心脏冲洗血液,然后用1 000ml含4%多聚甲醛,1%苦味酸和0.5%戊二醛的磷酸缓冲液(0.1mol/L PB,pH7.4)灌注固定。立即取出腰骶段脊髓,并在上述固定液内后固定3h(4℃),振动切片机切片,厚50μm,切片收集于磷酸盐缓冲液(0.01mol/L PBS,pH7.4)内。按ABC法进行免疫组织化学染色。切片在经PBS漂洗后依次移入:(1)豚鼠抗大鼠MOR的IgG(0.5mg/L)[9],室温孵育过夜;(2)结合有生物素的羊抗豚鼠IgG(Vector;1∶200),室温孵育3h;(3)ABC复合物(Vector;1∶200),室温孵育2h。其中,一抗和二抗用含0.01% Triton X-100,0.2%驴血清和0.03%NaN3的PBS稀释,ABC复合物用含0.01%Triton X-100的PBS稀释。最后切片在含0.05%DAB和0.003%H2O2的0.05%mol/L Tris-HCl缓冲液内呈色20~30min。切片经漂洗后在含1%锇酸的PB内室温下固定1h,梯度酒精及环氧丙烷脱水,Epon812平板包埋、聚合(45℃,24h;58℃,48h)。在解剖镜下取出后角浅层,LKB超薄切片机切片并裱于镍网上,然后进行GABA免疫组织化学染色,将镍网置于含兔抗大鼠GABA血清(1∶1 000)和0.01%Triton X-100的Tris-HCl缓冲液(TBST;pH7.6)液滴上,室温下湿盒内孵育24h。经pH7.6的TBST清洗后,再用pH8.2的TBST清洗,然后置于含结合有胶体金(直径10nm)颗粒的羊抗兔IgG(1∶40, British BioCell International)的液滴上,室温下湿盒内孵育3h。醋酸铀,枸橼酸铅染色,Hitachi100电子显微镜观察,摄片。
, 百拇医药
对照实验在省略一抗或用正常豚鼠血清或兔血清代替一抗的情况下进行。
结 果
用正常豚鼠血清替代或省略抗MOR抗体的条件下进行的对照实验显示,DAB呈色后的切片上只见有浅淡的背底,电镜下未观察到免疫反应阳性产物。包埋后GABA胶体金染色的对照实验亦在同样的条件(省略一抗或用正常兔血清替代)下进行,仅见到个别散在分布的胶体金颗粒。
与我们[12]先前的研究结果一致,猫脊髓后角浅层内MOR样免疫反应阳性产物主要分布于树突内,仅有少量的胞体及轴突呈现MOR阳性。在胞体内,阳性产物主要位于胞膜下,在胞浆内则主要位于粗面内质网的核糖体上。在树突内,阳性产物不仅出现于突触后膜下,亦分布于非突触部位的胞膜下,但前者多于后者(图1~3)。当1个神经终末内金颗粒数量大于其周围同等面积的非轴突结构内金颗粒数量的10倍以上者,则判定为GABA阳性[13]。GABA阳性轴突终末内含有多形性突触小泡,形成的突触为对称性突触(图1~3)。
, 百拇医药
双标电镜观察发现有相当数量的GABA阳性轴突终末与MOR阳性树突或胞体形成突触连接,其中多为轴树突触,偶见轴体突触,这些突触均为对称性突触(图1~4)。在所计数的100个有明确突触关系的MOR阳性树突和胞体中,其中有32个与GABA阳性轴突终末形成突触连接。实验中未发现有MOR阳性轴突含有GABA,也未见有MOR阳性的胞体为GABA阳性。
讨 论
MOR与吗啡的镇痛密切相关。MOR是通过与阿片类物质结合而发挥其生理效应。本研究结果表明,猫脊髓后角浅层内有约三分之一的MOR阳性的树突和胞体与GABA阳性终末形成突触连接,且全部为对称性突触。说明脊髓后角浅层内MOR神经元可能接受GABA能神经终末的抑制。以往的研究表明,脊髓后角浅层内约有70%的M-ENK阳性神经元同时含有GABA,提示M-ENK与GABA可能共存于轴突终末内[11]。此外,后角浅层神经元的膜片钳工作发现,GABA引起的氯电流可被MOR特异性的激动剂DAMGO增强[14]。因此,在伤害性刺激存在的情况下,后角浅层内GABA阳性轴突终末内的GABA可能与M-ENK一起释放,M-ENK作为内源性的吗啡类物质激活MOR阳性神经元而产生镇痛效应,而GABA可进一步增强M-ENK的镇痛效应。
, 百拇医药
此外,值得指出的是,本实验发现约三分之一的MOR阳性树突或胞体与GABA阳性终末之间存在突触联系,提示MOR阳性神经元除与GABA能神经终末形成突触外,可能还与含有其他神经活性物质的轴突终末形成突触。后角浅层内分布有大量不同来源且含有多种神经活性物质的神经终末,如5-羟色胺,降钙素基因相关肽,血管活性肠多肽等。这些神经活性物质均与伤害性刺激信息的传递或调制有着密切关系。MOR阳性神经元与这些神经活性物质之间的突触联系值得进一步探讨。
收稿1997-1 修回1997-07

图版说明
图1 示MOR阳性树突(De)与1个GABA能轴突终末(Ax)形成突触连接。箭头示突触活性点。标尺示0.2μm
图2 示MOR阳性树突(De)同时与2个GABA能轴突终末(Ax、Ax′)形成突触连接。箭头示突触点。标尺示0.3μm
, 百拇医药
图3 示MOR阳性树突(De)同时与1个GABA能轴突终末(Ax)及1个GABA阴性轴突终末(Ax′)形成突触连接。箭头示突触活性点。标尺示0.3μm
图4 示MOR阳性胞体(S)与1个GABA能轴突终末(Ax)形成突触连接。箭头示突触活性点。标尺示0.3μm
Explanation of figures
Fig.1 Showing synaptic contact between a MOR-positive dendrite(De)and a GABAergic axonal terminal(Ax).Arrow points to synaptic site.Bar=0.2μm
Fig.2 Showing a MOR-positive dendrite(De)makes synaptic contact with two GABAergic axonal terminals(Ax,Ax′).Arrows point to synaptic sites.Bar=0.3μm
, 百拇医药
Fig.3 Showing a MOR-positive dendrite(De)makes synaptic contacts with a GABAergic axonal
terminal(Ax)and a non-GABAergic axonal terminal(Ax′).Arrows point to synaptic sites.Bar=0.3μm
Fig.4 Showing synaptic contact between an MOR-positive cell body(S)and a GABAergic axonal terminal(Ax).Arrow points to synaptic site.Bar=0.3μm
* 国家自然科学基金资助课题(No.39600045)
参考文献
, http://www.100md.com
[1]Roberts E.γ-Aminbutyric acid and nervous system function.A perspective.Biochem Pharmac,1974,23(4):2639
[2]Todd AJ,McKenzie J.GABA-immunoreactive neuron in the dorsal horn of the rat spinal cord.Neuroscience,1989,31(3):799
[3]Cutting DA,Jordan CC.Alternative approaches to analgesia:Baclofer as a model compound.Bri J Pharmacol,1975,54(1):171
[4]Sawynok J.GABAergic mechanisms of analgesia:An update.Pharmocol Biochem and Behavior,1987,26(2):463
, http://www.100md.com
[5]Besson J-M,Chaouch A.Peripheral and spinal mechanisms of nociception.Physiol Rev,1987,67(1):67
[6]Mansour A,Waston SJ,Akil H.Opioid receptors:Past,present and future.Trends Neurosci,1995,18(1):69
[7]Atweh SF,Kuhar MJ.Autoradiographic localization of opiate receptor in rat brain.I spinal cord and lower medulla.Brain Res,1977,124(1):53
[8]Minami M,Onogi T,Toya T,et al.Molecular cloning and in situ hybridization histochemistry for rat μ-opioid receptor.Neurosci Res,1994,18(2):315
, 百拇医药
[9]Ding YQ,Kaneko T,Momura S,et al.Immunohistochemical localization of μ-opioid receptors in the central nervous sytem of the rat.J Comp Neurol,1996,367(1):375
[10]Raynor K,Kong H,Chen Y,et al.Pharmacological characteriztion of the cloned κ-,δ-,and μ-opioid receptors.Mol Pharmacol,1994,45(2):330
[11]Todd AJ,Spike RC,Russell G,et al.Immunohistochemical evidence that Met-Enkephalin and GABA coexist in some neurons in rat dorsal horn.Brain Res,1992,584(1):149
, http://www.100md.com
[12]王 丹,龚良维,丁玉强等.阿片μ受体在猫脊髓背角浅层内的超微定位分布研究.解剖学报,1998,29(1):30
[13]Phend KD,Weinberg RJ,Rustioni A.Techniques to optimize post-embedding single and double staining for amino acid neurotransmitters.J Histochem Cytochem,1992,40(4):1011
[14]Wang RA,Rardic M.Activation of μ-opioid receptor modulates BABAA receptor-mediated currents in isolated spinal horn neurons.Neurosci Lett,1994,180(1):109
, 百拇医药
THE SYNAPTIC RELATIONSHIP BETWEEN GABAERGIC AXON
TERMINALS AND NEURONS EXPRESSING μ-OPIOID
RECEPTOR IN THE SUPERFICIAL DORSAL
HORN OF THE CAT SPINAL CORD
Gong Liangwei,Ding Yuqiang△,Zheng Hengxing,Wang Dan,Qin Bingzhi,Li Jishuo
(Department of Anatomy and K.K.Leung Brain Research Center,Fourth Military Medical University,Xi'an)
, 百拇医药
The synaptic relationship between GABAergic axon terminals and neurons expressing μ-opioid receptor(MOR)in the superficial spinal dorsal horn of the cat was examined by using pre-embedding immunohistochemical method combined with post-embedding immunogold technique.About one third of the cell bodies and dendrites showing MOR-like immunoreactivity formed synaptic contacts with GABAergic axon terminals;all these synapses were symmetric.The present results suggest MOR-positive neurons in the superficial layers of the cat spinal cord may be modulated by GABA releasing from presynaptic axon terminals.
, 百拇医药
KEY WORDS μ-Opioid receptor;GABA;Electron microscopy;Immunohistochemistry;Spinal cord;Cat
△Department of Anatomy and K.K.Leung Brain Research Center,Fourth Military Medical University,Xi'an 710032,China
ULTRASTRUCTURAL LOCALIZATION OF SUBSTANCE P
RECEPTOR AND ITS SYNAPTIC RELATIONSHIP WITH
THE PRIMARY AFFERENTS IN THE SUPERFICIAL
, 百拇医药
DORSAL HORN OF THE RAT SPINAL CORD
Tao Yuanxiang,Li Yunqing*,Zhao Zhiqi
(Shanghai Brain Research Institute,Chinese Academy of Sciences,Shanghai 200031,China;
* Department of Anatomy,The Fourth Military Medical University,Xi'an 710032,China)
Abstract The ultrastructural localization of substance P receptor within the superficial dorsal horn of rat spinal cord was observed in the present study.Besides in dendritic profiles and cell bodies,substance P receptor-like immunoreactive(SPR-LI)products could first be found in axonal profiles in the superficial dorsal horn.By a combined methods of transganglionic transport of horseradish peroxidase conjugated with wheat-germ agglutinin(WGA-HRP)and immunocytochemistry,it was observed that some SPR-LI dendritic profiles received synaptic contacts from,or were apposed directly to,WGA-HRP-labeled primary afferent terminals in the superficial laminae.
, 百拇医药
KEY WORDS Substance P receptor;Primary afferent;Ultrastructure;Dorsal horn;Spinal cord;Rat
Introduction
The undecapeptide substance P(SP) has been widely studied as mediator of sensory information in the nervous system.In the spinal cord,SP and SP receptor(SPR) are mainly present in the superficial laminae of spinal dorsal horn[1-4],at the sites of small-diameter primary afferent termination,suggesting that SP and SPR may be involved in spinal nociceptive mechanism.Although the ultrasturctural localization of SPR within the superficial laminae has been reported in the previous studies[5],the detailed observation of SPR ultrastructural distribution is lacking.Since it has been known that many small-diameter primary afferent fibers contain SP[1],the neurons with SPR in the superficial laminae probably receive nociceptive information from small-diameter primary afferent fibers.However,morphological evidence is lacking.The present study was attempted to observe the detailed distribution of SPR at the electron microscopic level and the relationship between SPR-like immunoreactive(SPR-LI) neurons and the small-diameter primary afferent fibers in the superficial laminae of spinal cord by the methods of immunocytochemical staining of SPR and transganglionic transport of horseradish peroxidase conjugated with wheat-germ agglutinin(WGA-HRP).
, 百拇医药
Materials and methods
The experiment was performed on 14 male Sprague-Dawley rats(230-280g).The distribution of SPR-LI neurons in the spinal dorsal horn was examined in 6 rats.The rats were anesthetized deeply with sodium pentobarbital (60mg/kg,i.p.)and perfused transcardially with 100ml of 0.01mol/L phosphate-buffered saline(PBS,pH 7.4),followed by 400ml of a fixative containing 4% paraformaldehyde,0.5% glutaradehyde and 0.1% picric acid in 0.1mol/L phosphate buffer(PB,pH 7.4).The lumbar segment was removed,and cut into 50μm thick transverse sections on a vibrotome.According to the avidin-biotinylated peroxidase complex method[6],the sections were incubated in rabbit anti-SPR antiserum(1mg/L)(gift from Prof.N Mizuno) diluted in PBS containing 3% normal goat serum for 48h at 4℃,and in biotinylated goat anti-rabbit IgG(1∶200 Vector)for 2h at 37℃ and in avidin-biotin-peroxidase complex(1∶100 Vector),for 1 h at 37℃.The immunoreactivity was visualized by incubation in the Tris-HCl buffer containing 0.05% DAB and 0.01% hydrogen peroxide for 5-8min.Some sections were mounted on glass slides,dehydrated,cleared and coverslipped with OPX for light microscopy.
, 百拇医药
For electron microscopy,the rest of the SPR-immunostained sections were postfixed with 1% osmium tetraoxide in 0.1mol/L PB for 45min at room temperature,and dehydrated in graduated ethanol,and finally embedded in Epon.The ultrathin sections were counterstained with urany1 acetate and lead citrate.The observation was made with Phillips CM-100 electron microscope.
A combination of transganglionic transport of WGA-HRP and SPR immunocytochemistry was performed in the remaining 8 rats.Following anesthesia with sodium pentobarbital (40mg/kg,i.p.),the sciatic nerve was exposed above the knee and injected underneath the epineurial sheath with 2% WGA-HRP(1-1.5μl,Sigma).
, 百拇医药
Three days later,the animals were perfused.The sections were prepared as described above,and processed according to a tungstate/tetramethylbenzidine(TMB) method to reveal peroxidase[7]and stabilized with a 3.3-diaminobenzidine(DAB)/cobalt/H2O2 solution.Some sections were directly mounted onto glass slides to examine the distribution of WGA-HRP-labeled terminals.Rest of the sections were processed for the SPR immunocytochemistry as described above.
, http://www.100md.com
In the control experiment,when the primary antibody was omitted or replaced with normal rabbit serum,no immunoreactivity was detectable.
Results
A dense population of SPR-LI neurons were seen in lamina I,particularly in its medial part(Fig.1).The most common type of SPR-LI neurons had a fusiform cell body and an extensive rostrocaudal dendritic tree,which was typically confined to lamina I and the outer portion of lamina Ⅱ,and occasionally reached the inner portion of laminae Ⅱ and Ⅲ.Contrasting sharply with lamina Ⅰ,lamina Ⅱ contained very few SPR-LI neurons(Fig.1).However,it contained a significant population of processes that derived from SPR-LI neurons located in lamina Ⅰ and the deeper laminae of the dorsal horn.A few SPR-LI neurons were sparsely distributed in laminae Ⅲ-Ⅵ.Some of these neuronal processes extended dorsally into the superficial laminae.
, 百拇医药
The terminals labeled by WGA-HRP were confined to the superficial dorsal horn.The labeled terminals were more intense in lamina Ⅱ than in lamina Ⅰ(Fig.2).No terminals labeled by WGA-HRP were found in the rest areas of the dorsal horn.
Electron microscopic observation was made on the ultrathin sections obtained from the superficial dorsal horn.Cytoplasmic SPR-LI products were in granular or patch associated with the microtubules,rough endoplasmic reticulum,Golgi bodies and surface of mitochondria.A small number of SPR-LI cell bodies,which contained a large indented nucleus,were often observed in lamina Ⅰ(Fig.3).SPR-LI products were most often encountered in the dendritic profiles(Fig.4).The reaction products distributed along the dendritic membrane were not in a continuous fashion,and not only in postsynaptic sites,but also in non-synaptic areas.Synaptic contacts onto these SPR-LI dendrites were symmetrical or asymmetrical.The presynaptic terminals often exhibited numerous round clear vesicles and /or a few dense core ones.The dense core vesicles were located near or away from the junctional membrane(Fig.4).A direct apposition between two SPR-LI dendrites was found(Fig.5).As shown in Fig.6,SPR-LI products were also seen in the axonal profiles,which contained mitochondria and numerous clear vesicles.SPR-LI axons and terminals occasionally formed synaptic contacts with dendrites profiles.No glial profiles were found to contain immunoreactive products.
, 百拇医药
The highest density of WGA-HRP-labeled terminals was often observed in lamina Ⅱ.TMB reaction product was generally in the form of electron-dense crystals.Most of the labeled terminals in the superficial dorsal horn formed symmetric or asymmetric synapse with dendrites.Some formed the central element of complex glomerular arrangements(Fig.7).It was observed,particularly in lamina Ⅱ,that some WGA-HPR-labeled terminals contacted synaptically with(Fig.8),or apposed directly to,the SPR-LI dendrites.Of 260 SPR-LI dendritic profiles,153 were directly apposed to the terminals 34 of which were WGA-HRP-labeled terminals,and 96 received synaptic contacts from terminals 16 of which were WGA-HRP-labeled terminals.The remaining 11 SPR-LI dendrites were in close proximity to the neuroglia,vessels and unstained cell bodies.
, 百拇医药
Discussion
In the spinal cord,the distribution pattern of SPR-like immunoreactivities in the present study was in agreement with that in the previous studies[2,3].SPR-LI neurons were located mainly in lamina Ⅰ and very few in lamina Ⅱ.However,lamina Ⅱ contained some processes from SPR-LI neurons located in lamina Ⅰ and the deep laminae of the dorsal horn.The present result further showed that SPR-LI dendrities in the superficial laminae received synaptic junction with or direct apposition to WGA-HRP-labeled primary afferent terminals.This datum strongly provided a morphological substrate for spinal nociception mediated by NK-1 receptor(substance P preferring binding receptor)[8-11].
, 百拇医药
An important finding in the present ultrastructural observation was the existence of SPR-LI axonal terminals within the superficial dorsal horn.Although the origin of such SPR axonal terminals was unclear,the following results may give some hints.(1) Andoh et al reported that there was the expression of NK-1 receptor mRNA in the mouse dorsal root ganglion(DRG)neuron[12];(2) Our recent study showed NK-1 receptor mRNA expressed in xenopus oocyte following an injection of total mRNA extracted from cat DRG[13];(3) Carlton et al has demonstrated that SPR was associated with unmyelinated axons of rat glabrous skin[14].Taken together,it is conceivable that SPR-LI axonal terminals in the present study might stem from the central axon of the DRG neurons.SPR,likely as an autoreceptor,may be involved in modulation of release of transmitters from primary afferent terminals[15].Howerver,since there was no change of SPR in the spinal cord following the cutting of the dorsal root or the destroy of unmyelinated sensory fibers by capsaicin[2],the other origins of SPR-LI axonal terminals could not be ruled out.
, 百拇医药
The authors wish to thank Professor Chang H T and Li J S for their helpful criticism and revision of the manuscript,and Professor Mizuno N for giving us rabbit anti-SPR antiserum,and are grateful to Ms.Zhang M L and Wang D for electron microscopic and pholographic help.This work was supported in part by the Grant from the NNSFC.
Received Juny 1997

Explanation of figures
, 百拇医药
Fig.1 The distribution of SPR-positive neurons in the superficial dorsal horn.Scale bar:100μm
Fig.2 The distribution of WGA-HRP-labeled terminals in the superficial dorsal horn.Bar=100μm
Fig.3 The SPR-LI neuronal body with a indented nucleus(N)in lamina Ⅰ.Scale bar:1μm
Fig.4 SPR-LI dendrite(D)receiving synaptic contacts(arrows)from unstained terminals.Bar=0.25μm
, http://www.100md.com
Fig.5 A direct apposition(arrow)between two SPR-LI dendrites(D1,D2). bar=0.25μm
Fig.6 A SPR-LI axonal terminal(A).Bar=0.125μm
Fig.7 A WGA-HRP-labeled terminal (A) formed the central element of complex glomerulus arrangments in laminae Ⅱ.Bar=0.25μm
Fig.8 A synaptic contact(arrow)between a WGA-HRP-labeled terminal(A) and a SPR-LI dendrite(D).Bar=0.33μm
References
, http://www.100md.com
[1]Biasi S D,Rustioni A.Glutamate and substance P coexist in primary afferent terminals in the superficial laminae of spinal cord.Proc Nat1 Acad Sci U S A,1988,85(20):7820
[2]Brown J L,Liu H,Maggio J E,et al.,Morphological characterization of substance P receptor-immunoreactive neurons in the rat spinal cord and trigminal nucleus caudalis.J Comp Neurol,1995,356(3):327
[3]Nakaya Y,Kaneko,T.Shigemoto R,et al.Immunohistochemical localization of substance P receptor in the central nervous sytem of the adult rat.J Comp Neurol,1994,347(2):249
, 百拇医药
[4]Ruda M A.The pattern and place of nociceptive modulation in the dorsal horn.In:Yaksh T L(ed).Spinal Afferent Processing.New York,Plenum publishing corporation,1986,141-161
[5]Liu H,Brown J I,Jasmin L,et al.Synaptic relations between substance P and the substance P receptor:Light and electron microscopic characterization of the mismatch between neuropeptide and their receptor.Pro Nat1 Acad Sci USA,1994,91(3):1009
[6]Hsu S,Raine L,Fanger H A.A comparative study of the anti-peroxidase method and an avidinbiotin complex method for studying polypeptide hormones with radioimmunoassay antibodies.Am J Clin Pathol,1981,75(3):734
, 百拇医药
[7]Weinberg R J Van Eyck S L.A tertramethylbenzidine/tungstate reaction for horseradish peroxidase histochemistry.J Histochem Cytochem,1991,39(8):1143
[8]Murase K,Ryu P D,Randic M.Substance P augments a persistent slow inward calcium sensitive current in voltage-clamped rat spinal dorsal horn neurons of the rat.Brain Res,1986,365(2):369
[9]Murase K,Ryu P D,Randic M.Tachykinins modulate multiple ionic conductances in voltage-clamped rat spinal dorsal horn neurons.J Neurophysiol,1989,61(3):854
, http://www.100md.com
[10]Tao Y X,Wei F Zhao Z Q.A contribution of neurokinin-1 receptor to foramlin-induced c-fos expression in the rat spinal dorsal horn.Neurosci Lett,1997,221(2,3):105
[11]Wei F,Zhao Z Q.Blcokade of capsaicin-induced reduction of GABA-immunoreactivity by spantide in cat spinal superficial dorsal horn.Neuroscience,1996,71:(1):277
[12]Andoh T,Nagasawa T,Kuraishi Y.Expression of tachykinin NK1 receptor mRNA in dorsal root ganglia of the mouse.Mol Brain Res,1996,35(2):329
, 百拇医药
[13]Li H S,Bao Y D,Zhao Z Q.Expression of tachykinin receptor in Xenopus oocytes injected with poly(A)+RNA from cat dorsal root ganglion.Science in China Series C,1988,41(2):139
[14]Carlton S M,Zhou S,Coggeshall R E.Localization and activation of substance P receptor in unmyelinated axons of rat glabrous skin.Brain Res,1996,734(1):103
[15]Rusin K I,Ryu P D Randic M.Modulation of excitatory amino acid responses in rat dorsal horn neurons by tachykinins.J Neurophysiol,1992,68(2):265, 百拇医药