特比萘芬对小鼠胚胎的致畸作用
作者:卫敏 方几希
单位:卫敏 方几希 苏州医学院组织学胚胎学教研室,苏州 215007
关键词:特比萘芬;胚胎毒性;致畸作用
解剖学报990224 【摘要】 目的 研究特比萘芬对小鼠胚胎的致畸作用。 方法 将特比萘芬用225mg/kg、450mg/kg、900mg/kg3个剂量,于小鼠妊娠第6~15d,每天经口给药1次。 结果 3个剂量组孕鼠体重增长、着床数、活胎数、吸收胎数、死胎数无明显改变。900mg/kg、450mg/kg剂量组胎鼠体重、身长改变明显。225mg/kg剂量组胎鼠体重、身长无明显改变。3个剂量组胎鼠骨骼、外观和内脏均无异常发现。 结论 特比萘芬对小鼠无致畸作用。
MALFORMED EFFECT OF TERBINAFINE ON
, http://www.100md.com THE MOUSE EMBRYOS
Wei MinΔ, Fang Jixi
(Department of Histology and Embryology, Suzhou Medical College, Suzhou)
【Abstract】 Objective Investigating the malformed effect of terbinafine on the mouse embryos. Methods Terbinafine was divided into 225mg/kg, 450mg/kg and 900mg/kg groups. The mice were orally administered terbinafine from the 6th to 15th day after pregnancy once everyday. Results After the mice were given different doses of terbinafine, their weight, imbeded number, the number of living embryo, the number of absorbed embryo and the number of dead embryo did not change obviously. As the mice were given 900mg/kg and 450mg/kg of terbinafine, the weight and the body length of the embryo changed apparently. For this reason 900mg/kg and 450mg/kg of terbinafine have a slight inhibition on the mouse embryos growth. As the mice were given 225mg/kg of terbinafine, the weight and the body length of the embryo did not changed apparently. After the pregnant mice were given different doses of terbinafine, the abnormality in statistics was not found in the skeleton of the mouse embryos, the exterior and the internal organs.Conclusions Terbinafine has no malformed effect on the mouse embryos.
, 百拇医药
【Key words】 Terbinafine; Embryo toxicity; Malformation
特比萘芬(terbinafine)是90年代发展的一种丙烯胺类抗真菌药,通过特异性抑制真菌角鲨烯环氧化酶,导致前体角鲨烯大量积聚及胞壁麦角固醇合成受抑而对真菌起抑杀作用[1]。由于该药抗菌谱广、表皮高渗透性、亲脂性及其杀菌性,较其他抗真菌药优越[2~4],临床应用中亦证明可大大提高对某些真菌病的疗效。实验研究表明,目前应用广泛的抗真菌药酮康唑(ketoconazole)、灰黄霉素(griseofulvin)在动物均可产生先天性畸形[5],伊曲康唑(itraconazole)在临床应用中亦有“孕妇慎用”的警句。为了弄清特比萘芬对胚胎发育是否有影响,我们对该药的致畸胎作用进行了研究。
材料和方法
用苏州医学院实验动物中心提供的25~30g昆明种小鼠,雄鼠100只,雌鼠200只。在每个标准塑料饲养盒内饲养5只。小鼠食标准固体饲料,自由饮水。室温22±1℃,湿度55±5%,采用12:12h明暗光照,定时通风。每3d更换1次垫底木屑。小鼠饲养2周后雌、雄(2∶1)同笼,每日晨检阴栓,查到阴栓之日定为妊娠第0d。阴栓阳性鼠随机分组,每组25只。共3组作为给药组。特比萘芬(批号:970129,山东齐鲁制药厂生产)剂量分组以LD50的1/4为高剂量组900mg/(kg.d);中剂量组取高剂量组的1/2,即450mg/(kg.d);低剂量组取高剂量组的1/4即225mg/(kg.d)。用2%明胶作为溶剂把特比萘芬配成不同浓度的混悬液。另取孕鼠25只用2%明胶溶剂作为阴性对照。以上4组于母鼠妊娠第6~15d(胚胎器官形成期)经口给药1次/d,给药体积为0.25ml/10g体重)。用环磷酰胺(cyclophosphamide,批号:901217,上海第十二制药厂生产)10mg/(kg.d)于母鼠妊娠第8~12d腹腔注射作为阳性对照(表1)。
, 百拇医药
表1 特比萘芬的剂量分组及各组孕鼠数
Table 1 Doses of terbinafine and number of pregnant mouse 组别mg/(kg.d)group
孕鼠数
pregnant mouse number
体重(g)
weight
900
25
27.09±2.87
450
, 百拇医药
25
27.24±2.91
225
25
26.88±3.03
阴性对照组
negative control
25
26.70±2.88
阳性对照组
positive control
25
, 百拇医药
27.06±3.28
妊娠期间每3d称母鼠体重1次。于妊娠第18d处死解剖各组母鼠,观察母鼠内脏器官和妊娠情况,记录活胎数、死胎数、吸收胎数和总着床数。将胎鼠称重、测量身长并检查外观畸形。然后把半数活胎鼠用Bouin固定1周后做徒手切片检查内脏;另半数用95%乙醇固定1周后做骨骼畸形检查。
实验数据统计方法:平均体重、平均着床数、平均活胎数、平均死胎数、平均吸收胎数、平均活胎体重用t检验;畸胎率、死亡率用χ2检验。结果和讨论
给药期间各组均有个别孕鼠死亡,各组孕鼠体重增长与阴性对照组比较无显著性差异(P>0.05),而与阳性对照组比较有非常显著性差异(P<0.01),见表2。
表2 特比萘芬对孕鼠的影响
Table 2 The influence on pregnant mouse
, 百拇医药
treated by terbinafine 组别mg/(kg*d)group
孕鼠数
pregnant mouse
number
死亡孕鼠数
dead pregnant
mouse number
体重增长(g)
increase of
weight
900
, 百拇医药
25
4
15.95±2.78
450
25
3
16.31±3.65
225
25
2
16.65±2.82
阴性对照组
negative control
, http://www.100md.com
25
2
16.99±2.48*
阳性对照组
positive control
25
5
6.39±3.69**
*与阴性对照组相比(As compared with negative control group)P>0.05
**与阳性对照组相比(As compared with positive control group)P<0.01.
, 百拇医药
3个剂量组小鼠的平均着床数、平均活胎数、平均吸收胎数、平均死胎数与阴性对照组比较无显著性差异(P>0.05),而与阳性对照组比较则有非常显著性差异(P<0.01),见表3。表3 特比萘芬对小鼠胚胎的影响
Table 3 The influence on mouse embryo treated by terbinafine 组别mg/(kg.d)group
着床数
imbeded number
吸收胎数
absorbed embryo number
活胎数
living embryo number
, http://www.100md.com
死胎数
dead embryo number
P
900
11.01±1.20
0.3±0.18
10.4±1.60
0.2±0.81
>0.05*
450
11.01±1.10
0.2±0.11
, 百拇医药
11.1±1.40
0.2±0.66
>0.05*
225
12.01±0.40
0.1±0.02
11.5±1.00
0.2±0.08
>0.05*
阴性对照组
negative control
, 百拇医药
12.04±0.20
0.1±0.01
11.5±1.10
0.2±0.06
阳性对照组
positive control
9.80±1.00
0.8±0.50
4.8±3.18
3.8±2.11
<0.01**
, 百拇医药 *与阴性对照组相比(As compared with negative control group).
**与阳性对照组相比(As compared with positive control group).
900mg/kg剂量组与450mg/kg剂量组的胎鼠体重、身长与阴性对照组比较均有显著性差异(P<0.05);而225mg/kg剂量组的胎鼠体重、身长与阴性对照组比较无显著性差异(P>0.05)。但3个剂量组的胎鼠体重、身长与阳性对照组的比较均有非常显著性差异(P<0.01),见表4。
对胎鼠骨骼系统检查结果,阳性对照组的胎鼠均有枕骨、颈椎和胸骨的骨化不全或缺失,占受检总数的100%。900mg/kg剂量组有枕骨发育不全(2/102)及胸骨骨化不全(1/102)等骨骼异常,但畸形率均在正常范围,与阴性对照组比较无显著性差异(P>0.05),而与阳性对照组比较有非常显著性差异(P<0.01)。450mg/kg剂量组和225mg/kg剂量组的胎鼠均无骨骼畸形发现。阳性对照组26只胎鼠均有外观畸形,其中有脑外露3只,脑膨出23只。阴性对照组和3个剂量组均未观察到胎鼠外观畸形或内脏畸形。表4 特比萘芬对胎鼠生长的影响
, 百拇医药
Table 4 The influence on mouse fetus growth
treated by terbinafine 组别mg/(kg.d)group
仔鼠数(只)
fetus
number
体重(g)
weight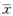 ±s
±s
身长(mm)
length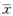 ±s
±s
, 百拇医药
P
900
204
1.34±1.04
29.8±2.41
<0.05*
450
236
1.38±1.11
30.7±1.23
<0.05*
225
, 百拇医药
242
1.43±1.01
31.1±1.33
>0.05*
阴性对照组
negative control
244
1.44±1.02
31.7±1.20
阳性对照组
positive control
26
, 百拇医药
0.96±0.28
28.88±1.61
<0.01**
*与阴性对照组相比(As compared with negative control group).
**与阳性对照组相比(As compared with positive control group).
本实验对特比萘芬的胚胎毒性作用进行了研究。环磷酰胺阳性对照组引起小鼠明显的外观及骨骼畸形,表明选用的小鼠对致畸剂反应敏感。实验组最大给药剂量为900mg/kg,单次剂量相当于1/4 LD50,累积给药达2.5LD50(mg/kg)。结果显示:除900mg/kg、450mg/kg剂量对胎鼠生长、发育产生轻度抑制作用并导致小鼠胚胎宫内生长迟缓外,其余各项指标与阴性对照组比较均无显著性差异。3个剂量组的各项指标与环磷酰胺阳性对照组比较均有非常显著性差异。3个剂量组胎鼠骨骼、外观和内脏的致畸变化均无统计学意义。提示在我们选用的剂量范围和实验条件下,特比萘芬对小鼠未见到致畸作用。
, 百拇医药
收稿1997-11 修回1998-09 参考文献
[1]Balfour J A, Faulds D. Terbinafine: A review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential in superficial mycoses. Drugs, 1992, 43(2):259
[2]Zaias N. Management of onychomycosis with oral terbinafine. J Am Acad Dermatol, 1990, 23(4 Pt 2):810
[3]Zaias N, Serrano L. The successful treatment of finger trichophyton rubrum onychomycosis with oral terbinafine. Clin Exp Dermatol, 1989, 14(2):120
[4]Savin R C. Oral terbinafine versus griseofulvin in the treatment of moccasin-type tinea pedis. J Am Acad Dermatol, 1990, 23(4 Pt 2):807
[5]Bechter R, Schmid B, Mayer F K. Teratogenic potential of antimycotic drugs evaluated in the whole-embryo culture system. Food Chem Toxicol, 1989, 24(6-7):641, 百拇医药
单位:卫敏 方几希 苏州医学院组织学胚胎学教研室,苏州 215007
关键词:特比萘芬;胚胎毒性;致畸作用
解剖学报990224 【摘要】 目的 研究特比萘芬对小鼠胚胎的致畸作用。 方法 将特比萘芬用225mg/kg、450mg/kg、900mg/kg3个剂量,于小鼠妊娠第6~15d,每天经口给药1次。 结果 3个剂量组孕鼠体重增长、着床数、活胎数、吸收胎数、死胎数无明显改变。900mg/kg、450mg/kg剂量组胎鼠体重、身长改变明显。225mg/kg剂量组胎鼠体重、身长无明显改变。3个剂量组胎鼠骨骼、外观和内脏均无异常发现。 结论 特比萘芬对小鼠无致畸作用。
MALFORMED EFFECT OF TERBINAFINE ON
, http://www.100md.com THE MOUSE EMBRYOS
Wei MinΔ, Fang Jixi
(Department of Histology and Embryology, Suzhou Medical College, Suzhou)
【Abstract】 Objective Investigating the malformed effect of terbinafine on the mouse embryos. Methods Terbinafine was divided into 225mg/kg, 450mg/kg and 900mg/kg groups. The mice were orally administered terbinafine from the 6th to 15th day after pregnancy once everyday. Results After the mice were given different doses of terbinafine, their weight, imbeded number, the number of living embryo, the number of absorbed embryo and the number of dead embryo did not change obviously. As the mice were given 900mg/kg and 450mg/kg of terbinafine, the weight and the body length of the embryo changed apparently. For this reason 900mg/kg and 450mg/kg of terbinafine have a slight inhibition on the mouse embryos growth. As the mice were given 225mg/kg of terbinafine, the weight and the body length of the embryo did not changed apparently. After the pregnant mice were given different doses of terbinafine, the abnormality in statistics was not found in the skeleton of the mouse embryos, the exterior and the internal organs.Conclusions Terbinafine has no malformed effect on the mouse embryos.
, 百拇医药
【Key words】 Terbinafine; Embryo toxicity; Malformation
特比萘芬(terbinafine)是90年代发展的一种丙烯胺类抗真菌药,通过特异性抑制真菌角鲨烯环氧化酶,导致前体角鲨烯大量积聚及胞壁麦角固醇合成受抑而对真菌起抑杀作用[1]。由于该药抗菌谱广、表皮高渗透性、亲脂性及其杀菌性,较其他抗真菌药优越[2~4],临床应用中亦证明可大大提高对某些真菌病的疗效。实验研究表明,目前应用广泛的抗真菌药酮康唑(ketoconazole)、灰黄霉素(griseofulvin)在动物均可产生先天性畸形[5],伊曲康唑(itraconazole)在临床应用中亦有“孕妇慎用”的警句。为了弄清特比萘芬对胚胎发育是否有影响,我们对该药的致畸胎作用进行了研究。
材料和方法
用苏州医学院实验动物中心提供的25~30g昆明种小鼠,雄鼠100只,雌鼠200只。在每个标准塑料饲养盒内饲养5只。小鼠食标准固体饲料,自由饮水。室温22±1℃,湿度55±5%,采用12:12h明暗光照,定时通风。每3d更换1次垫底木屑。小鼠饲养2周后雌、雄(2∶1)同笼,每日晨检阴栓,查到阴栓之日定为妊娠第0d。阴栓阳性鼠随机分组,每组25只。共3组作为给药组。特比萘芬(批号:970129,山东齐鲁制药厂生产)剂量分组以LD50的1/4为高剂量组900mg/(kg.d);中剂量组取高剂量组的1/2,即450mg/(kg.d);低剂量组取高剂量组的1/4即225mg/(kg.d)。用2%明胶作为溶剂把特比萘芬配成不同浓度的混悬液。另取孕鼠25只用2%明胶溶剂作为阴性对照。以上4组于母鼠妊娠第6~15d(胚胎器官形成期)经口给药1次/d,给药体积为0.25ml/10g体重)。用环磷酰胺(cyclophosphamide,批号:901217,上海第十二制药厂生产)10mg/(kg.d)于母鼠妊娠第8~12d腹腔注射作为阳性对照(表1)。
, 百拇医药
表1 特比萘芬的剂量分组及各组孕鼠数
Table 1 Doses of terbinafine and number of pregnant mouse 组别mg/(kg.d)group
孕鼠数
pregnant mouse number
体重(g)
weight
900
25
27.09±2.87
450
, 百拇医药
25
27.24±2.91
225
25
26.88±3.03
阴性对照组
negative control
25
26.70±2.88
阳性对照组
positive control
25
, 百拇医药
27.06±3.28
妊娠期间每3d称母鼠体重1次。于妊娠第18d处死解剖各组母鼠,观察母鼠内脏器官和妊娠情况,记录活胎数、死胎数、吸收胎数和总着床数。将胎鼠称重、测量身长并检查外观畸形。然后把半数活胎鼠用Bouin固定1周后做徒手切片检查内脏;另半数用95%乙醇固定1周后做骨骼畸形检查。
实验数据统计方法:平均体重、平均着床数、平均活胎数、平均死胎数、平均吸收胎数、平均活胎体重用t检验;畸胎率、死亡率用χ2检验。结果和讨论
给药期间各组均有个别孕鼠死亡,各组孕鼠体重增长与阴性对照组比较无显著性差异(P>0.05),而与阳性对照组比较有非常显著性差异(P<0.01),见表2。
表2 特比萘芬对孕鼠的影响
Table 2 The influence on pregnant mouse
, 百拇医药
treated by terbinafine 组别mg/(kg*d)group
孕鼠数
pregnant mouse
number
死亡孕鼠数
dead pregnant
mouse number
体重增长(g)
increase of
weight
900
, 百拇医药
25
4
15.95±2.78
450
25
3
16.31±3.65
225
25
2
16.65±2.82
阴性对照组
negative control
, http://www.100md.com
25
2
16.99±2.48*
阳性对照组
positive control
25
5
6.39±3.69**
*与阴性对照组相比(As compared with negative control group)P>0.05
**与阳性对照组相比(As compared with positive control group)P<0.01.
, 百拇医药
3个剂量组小鼠的平均着床数、平均活胎数、平均吸收胎数、平均死胎数与阴性对照组比较无显著性差异(P>0.05),而与阳性对照组比较则有非常显著性差异(P<0.01),见表3。表3 特比萘芬对小鼠胚胎的影响
Table 3 The influence on mouse embryo treated by terbinafine 组别mg/(kg.d)group
着床数
imbeded number
吸收胎数
absorbed embryo number
活胎数
living embryo number
, http://www.100md.com
死胎数
dead embryo number
P
900
11.01±1.20
0.3±0.18
10.4±1.60
0.2±0.81
>0.05*
450
11.01±1.10
0.2±0.11
, 百拇医药
11.1±1.40
0.2±0.66
>0.05*
225
12.01±0.40
0.1±0.02
11.5±1.00
0.2±0.08
>0.05*
阴性对照组
negative control
, 百拇医药
12.04±0.20
0.1±0.01
11.5±1.10
0.2±0.06
阳性对照组
positive control
9.80±1.00
0.8±0.50
4.8±3.18
3.8±2.11
<0.01**
, 百拇医药 *与阴性对照组相比(As compared with negative control group).
**与阳性对照组相比(As compared with positive control group).
900mg/kg剂量组与450mg/kg剂量组的胎鼠体重、身长与阴性对照组比较均有显著性差异(P<0.05);而225mg/kg剂量组的胎鼠体重、身长与阴性对照组比较无显著性差异(P>0.05)。但3个剂量组的胎鼠体重、身长与阳性对照组的比较均有非常显著性差异(P<0.01),见表4。
对胎鼠骨骼系统检查结果,阳性对照组的胎鼠均有枕骨、颈椎和胸骨的骨化不全或缺失,占受检总数的100%。900mg/kg剂量组有枕骨发育不全(2/102)及胸骨骨化不全(1/102)等骨骼异常,但畸形率均在正常范围,与阴性对照组比较无显著性差异(P>0.05),而与阳性对照组比较有非常显著性差异(P<0.01)。450mg/kg剂量组和225mg/kg剂量组的胎鼠均无骨骼畸形发现。阳性对照组26只胎鼠均有外观畸形,其中有脑外露3只,脑膨出23只。阴性对照组和3个剂量组均未观察到胎鼠外观畸形或内脏畸形。表4 特比萘芬对胎鼠生长的影响
, 百拇医药
Table 4 The influence on mouse fetus growth
treated by terbinafine 组别mg/(kg.d)group
仔鼠数(只)
fetus
number
体重(g)
weight
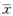 ±s
±s身长(mm)
length
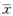 ±s
±s, 百拇医药
P
900
204
1.34±1.04
29.8±2.41
<0.05*
450
236
1.38±1.11
30.7±1.23
<0.05*
225
, 百拇医药
242
1.43±1.01
31.1±1.33
>0.05*
阴性对照组
negative control
244
1.44±1.02
31.7±1.20
阳性对照组
positive control
26
, 百拇医药
0.96±0.28
28.88±1.61
<0.01**
*与阴性对照组相比(As compared with negative control group).
**与阳性对照组相比(As compared with positive control group).
本实验对特比萘芬的胚胎毒性作用进行了研究。环磷酰胺阳性对照组引起小鼠明显的外观及骨骼畸形,表明选用的小鼠对致畸剂反应敏感。实验组最大给药剂量为900mg/kg,单次剂量相当于1/4 LD50,累积给药达2.5LD50(mg/kg)。结果显示:除900mg/kg、450mg/kg剂量对胎鼠生长、发育产生轻度抑制作用并导致小鼠胚胎宫内生长迟缓外,其余各项指标与阴性对照组比较均无显著性差异。3个剂量组的各项指标与环磷酰胺阳性对照组比较均有非常显著性差异。3个剂量组胎鼠骨骼、外观和内脏的致畸变化均无统计学意义。提示在我们选用的剂量范围和实验条件下,特比萘芬对小鼠未见到致畸作用。
, 百拇医药
收稿1997-11 修回1998-09 参考文献
[1]Balfour J A, Faulds D. Terbinafine: A review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential in superficial mycoses. Drugs, 1992, 43(2):259
[2]Zaias N. Management of onychomycosis with oral terbinafine. J Am Acad Dermatol, 1990, 23(4 Pt 2):810
[3]Zaias N, Serrano L. The successful treatment of finger trichophyton rubrum onychomycosis with oral terbinafine. Clin Exp Dermatol, 1989, 14(2):120
[4]Savin R C. Oral terbinafine versus griseofulvin in the treatment of moccasin-type tinea pedis. J Am Acad Dermatol, 1990, 23(4 Pt 2):807
[5]Bechter R, Schmid B, Mayer F K. Teratogenic potential of antimycotic drugs evaluated in the whole-embryo culture system. Food Chem Toxicol, 1989, 24(6-7):641, 百拇医药