睾丸摘除后大鼠垂体前叶CGRP免疫反应神经纤维的变化
作者:卢春蓉 孟繁东 王浩军 鞠躬
单位:卢春蓉 孟繁东 王浩军 鞠躬(第四军医大学神经科学研究所,西安 710032)
关键词:垂体前叶;CGRP;神经纤维;睾丸摘除;大鼠
解剖学报990304 【摘要】 目的 探讨体内睾酮水平变化对大鼠垂体前叶CGRP免疫反应神经纤维的影响。 方法 成年雄性SD大鼠随机分为7个组:正常对照组(NOR);睾丸摘除组(OX);假手术组(SO);正常+丙酸睾丸素组(NOR+TP);睾丸摘除+丙酸睾丸素组(OX+TP);正常+油剂组(NOR+V);睾丸摘除+油剂组(OX+V)。采用免疫组织化学和图像分析方法观察大鼠垂体前叶中CGRP免疫反应神经纤维的变化。 结果 睾丸摘除7d后,大鼠垂体前叶CGRP免疫反应神经纤维发生了显著变化,表现为膨体型纤维较正常组稠密,分支增多,迂曲成片,盘绕成网,分布更加广泛,膨体数量显著增多;粗纤维较正常组粗,且发出许多迂曲盘绕的细纤维,膨体形态变大。t检验显示,OX组与NOR组垂体前叶内CGRP免疫反应神经纤维的平均面积密度差异显著,OX+V与NOR组差异显著。而SO组、NOR+TP组、OX+TP组、NOR+V组与NOR组比较,CGRP免疫反应神经纤维的平均面积密度无显著差异。与NOR组比较,NOR+TP组、OX+TP组中,垂体前叶CGRP免疫反应神经纤维的形态以细纤维为主。 结论 垂体前叶中CGRP免疫反应神经纤维对体内睾酮水平的剧烈变化能作出积极反应,从功能形态学上证明了这些神经纤维对前叶腺细胞的分泌可起一定的调节作用。
, http://www.100md.com
THE CHANGES OF CALCITONIN GENE-RELATED PEPTIDE
IMMUNOREACTIVE NERVE FIBERS IN THE ANTERIOR
PITUITARY OF THE RAT FOLLOWING ORCHIECTOMY
Lu Chunrong, Meng Fandong, Wang Haojun, Ju GongΔ
(Institute of Neurosciences,The Fourth Military Medical University, Xi'an)
【Abstract】 Objective The present study was aimed at investigating the effects of the changes of testosterone levels on the calcitonin gene-related peptide(CGRP)-immunoreactive nerve fibers in the anterior pituitary(AP) of the rat.Methods The adult male SD rats were randomly divided into 7 groups: (1)normal(NOR), (2)orchiectomy(OX), (3)shame operation(SO), (4)normal+testosterone propionate(NOR+TP), (5)orchiectomy+testosterone propionate(OX+TP), (6)normal+vehicle(NOR+V), (7)orchiectomy+vehicle(OX+V). Immunohistochemistry and image analysis were used to study the changes of CGRP-immunoreactive nerve fibers in the AP of the rat.Results Following orchiectomy 7d,the amount of CGRP-immunoreactive nerve fibers in the AP of the rat increased significantly, with the tortuous varicose fibers more densely distributed than that of NOR and the amount of varicosities increased significantly, while the thick fibers were more thicker than that of NOR,and many thin fibers were sent out from the thick fibers, and the varicosities were more larger. Student's t-test showed that the difference of the average area density of the CGRP-immunoreactive nerve fibers in the AP between OX and NOR was significant. The difference of that between OX+V and NOR was also significant. But the difference of that between SO,NOR+TP,OX+TP,NOR+V and NOR was insignificant, whereas the appearance of the CGRP-immunoreactive nerve fibers of NOR+TP and OX+TP was different from that of NOR, with the former two had more thin fibers and less thick fibers.Conclusion The results demonstrated that CGRP-immunoreactive nerve fibers in the AP of the rat could respond actively to the violent change of circulating TP, suggesting that CGRP-immunoreactive nerve fibers might regulate secretion of the AP gland cells.
, 百拇医药
【Key words】 Anterior pituitary; Calcitonin gene-related peptide; Nerve Fibers; Orchiectomy; Rat
近年来,在一些哺乳动物垂体前叶中陆续发现了一定数量的SP及CGRP免疫反应神经纤维的分布[1~7]。免疫电镜研究发现犬垂体前叶CGRP免疫反应神经纤维与各种腺细胞联系密切,并可与ACTH细胞、GH细胞形成典型的不对称的突触结构[8],推测该神经纤维可能对ACTH、GH的合成与分泌具有调节作用。功能形态学和激素替代实验表明,对大鼠行双侧肾上腺摘除术后,垂体前叶中的CGRP免疫反应神经纤维数量显著增多,说明CGRP免疫反应神经纤维对机体的内分泌状态的变化产生了积极的反应[9],提示CGRP免疫反应神经纤维可能对垂体前叶的分泌发挥一定的调节作用。本研究观察了睾丸摘除后,大鼠体内雄性激素水平剧烈变化时,大鼠垂体前叶中CGRP免疫反应神经纤维的变化。
, 百拇医药
材料和方法
1. 动物
成年雄性SD大鼠56只,体重200~230g,由第四军医大学动物实验研究中心提供。随机分为7个组,每组8只:(1)正常对照组(NOR),(2)双侧睾丸摘除组(OX),(3)假手术组(SO),(4)正常+丙酸睾丸素组(NOR+TP),(5)睾丸摘除+丙酸睾丸素组(OX+TP),(6)正常+油剂组(NOR+V),(7)睾丸摘除+油剂组(OX+V)。
丙酸睾丸素的给药量为每日每只130μg(皮下注射),溶于芝麻油(0.2ml/只),于睾丸摘除次日开始给药。正常给药时间与摘除组给药时间同步,给药时间为每日17:30~18:00。给油剂时间与给药时间同步。饲养条件为12h光照(8:30~20:30),12h黑暗。给予足够的食物和饮水。
2. 取材
, 百拇医药
睾丸摘除后7d取材,取材时间为上午9:00~11:00,经升主动脉灌注100ml生理盐水及新鲜配制的4%多聚甲醛PB溶液(0.1mol/L,pH7.4)500ml,4℃,1h后取出垂体,在4℃、4%多聚甲醛PB溶液中后固定2~4h,入20%蔗糖PB溶液中,4℃过夜。为保证所有动物均在同一时间固定,每组实验相差1d。
3. 免疫组织化学方法
恒冷箱连续矢状切片,片厚30μm ,贴片于经1%明胶+0.05%硫酸铬钾处理过的载玻片上;在空气中干燥15min后,入0.01mol/L的KPBS(pH7.4),经100%甲醇+0.3%H2O2封闭内源性过氧化物酶,KPBS浸洗3×5min,浸泡于0.3%Triton X-100中30min,切片入兔抗CGRP血清(1:4 000,美国Salk Institute,W.Vale博士惠赠),抗体稀释液中含1%BSA、0.3%Triton X-100、0.02%NaN3,4℃孵育48h。KPBS浸洗3×10min,入结合生物素的羊抗兔第二抗体中室温孵育2~4h(1∶200,ABC Kit,Sigma),KPBS浸洗3×10min,入ABC复合物(1:200)室温孵育2~4h。葡萄糖氧化酶-DAB-硫酸镍胺法显色。常规脱水透明,DPX封片。对照实验用KPBS代替第一抗体,结果为阴性。
, 百拇医药
4. 图像分析
对每套切片随机等距抽取10张切片。在抽取的每张切片中,在×20物镜下再等距随机抽取5个视野,在图像分析仪Quantimat 570C上,测视野中CGRP免疫反应神经纤维的相对面积密度。计算50个视野中CGRP阳性神经纤维的总平均面积密度,对各实验组和对照组的相对平均面积密度进行团体t检验。
根据体视学卡瓦列里原理,垂体前叶的体积以其截面积来估算,即对每套切片等距抽取10张切片,测切片上前叶的面积,以其面积总和估算其体积值。t检验显示各组垂体前叶的体积之间无明显差别(P>0.25)。
结果
与正常对照组比,睾丸摘除7d后,大鼠垂体前叶CGRP免疫反应神经纤维中,其膨体型细纤维及粗纤维均发生变化,表现为:(1)膨体型纤维较正常组稠密,分布更加广泛,分支增多,成片迂曲盘绕成网,与腺细胞关系密切(图1,2)。(2)粗纤维较正常组粗,且发出许多迂曲盘绕的细纤维,可见其上大小不等的膨体,纤维分支处膨体形态较大(图3,4)。假手术组、正常+油剂组与正常对照组比,纤维平均面积密度无明显差异。摘除+油剂组与摘除组之间纤维平均面积密度也无明显差异,各组间纤维平均面积密度见附表。
, 百拇医药
附表 睾丸摘除后各组动物垂体前叶CGRP免疫反应神经纤维平均面积密度
Table Sample averaged area density of the CGRP-
immunoreactive nerve fibers in the anterior pituitaries of the rats following orchiectomy 动物分组
groups
平均面积密度(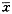 ±s)
±s)
averaged area density
正常对照组(NOR)
, 百拇医药
0.0024±0.00055
假手术组(SO)
0.0024±0.00053
睾丸摘除组(OX)
0.0074±0.0011
正常+丙酸睾丸素组(NOR+TP)
0.0024±0.00055
睾丸摘除+丙酸睾丸素组(OX+TP)
0.0020±0.00023
正常+油剂组(NOR+V)
, 百拇医药
0.0026±0.00047
睾丸摘除+油剂组(OX+V)
0.0066±0.00058
与NOR组比:n=5,P<0.001 compared with NOR:n=5,**P<0.001
睾丸摘除+丙酸睾丸素组与正常对照组比较,纤维类型以膨体型细纤维为主,膨体较多。正常+丙酸睾丸素组与正常对照组比较,粗而直的纤维很少,大多为分布于垂体前叶内部中央,多弯曲、多膨体的细纤维。上述两组与正常组比较,纤维平均面积密度无明显差异。
讨论
有关睾酮与垂体前叶内CGRP合成的关系,前人作了一些初步的研究。如Gon等[10]发现大鼠垂体前叶内促性腺激素细胞合成、释放CGRP,当雄性大鼠去势后,垂体前叶CGRP阳性细胞数量显著增加,补充睾丸酮则能反转这种效应。该实验结果提示体内睾酮水平可能调控垂体前叶内促性腺激素细胞CGRP的表达,或者说在某种程度上睾酮可能抑制垂体前叶CGRP的表达。
, http://www.100md.com
除了垂体前叶内促性腺激素细胞能合成、释放CGRP之外,在正中隆起处还分布许多CGRP阳性神经纤维终末。这些CGRP纤维可能起源于下丘脑室周核,此部位均含有CGRP阳性细胞体[7]。雌雄鼠的视前区CGRP阳性细胞群存在显著性分化,雌鼠的内侧视前核中的CGRP阳性细胞比雄鼠中高20倍,而睾酮对于这种性分化是必需的。睾酮也对成年雄鼠的内侧视前核的CGRP阳性细胞发挥一种持续性的、潜在的抑制影响[11],有关这种抑制作用的机制尚不清楚。
CGRP能否在正中隆起处释放,通过垂体门脉系统,以类似下丘脑促垂体因子的方式作用于垂体,目前还不清楚。尽管有人未能在大鼠垂体前叶发现CGRP的受体,而后续研究报道大鼠垂体前叶有CGRP的特异结合位点。[12]。由于垂体前叶细胞能合成、释放CGRP,CGRP也可能从正中隆起通过垂体门脉系统到达垂体前叶,CGRP对垂体功能的作用即引起人们的关注。业已有一些研究认为CGRP可影响在体或离体下垂体前叶的分泌活动,如较低浓度的CGRP抑制离散培养的垂体前叶细胞分泌GH,而较高浓度的CGRP则刺激GH、PRL释放[13]。在支配垂体前叶的神经纤维中含有CGRP,CGRP可以调控垂体前叶的激素分泌,但CGRP阳性神经纤维在其中发挥什么作用,有待进一步研究。
, http://www.100md.com
本研究结果显示,睾丸摘除7d后,大鼠垂体前叶中CGRP阳性神经纤维数量显著增加,纤维变粗,纤维分支增多,膨体形态变大,数目增多。若在睾丸摘除后每日补充一定量的睾酮则能逆转这种变化,说明前叶中CGRP阳性神经纤维对体内睾酮水平的剧烈变化能作出反应。睾酮可作用于垂体和下丘脑水平,通过负反馈调节促性腺激素的分泌。睾丸摘除后,垂体前叶中FSH和LH含量及释放均增加。本实验结果说明,睾酮也反馈作用于支配垂体前叶的CGRP阳性神经元,影响其神经纤维中CGRP的含量。提示前叶中CGRP阳性神经纤维可能调节促性腺激素的分泌。
正常大鼠每日注射一定量的睾酮,导致体内睾酮水平升高时,与正常对照组比较,垂体前叶CGRP阳性神经纤维的平均面积密度一致,但两者神经纤维的形态分布却有较大的差异。推测可能的原因为:垂体前叶CGRP阳性神经纤维对体内睾酮水平降低或升高的反应程度不同;体内高水平睾酮可能影响了CGRP由粗纤维向细纤维的转运。
本实验结果与肾上腺摘除后大鼠垂体前叶中CGRP纤维的变化情况较相似[9],提示垂体前叶中CGRP阳性神经纤维可能对垂体前叶的功能发挥某些调控作用,从而为鞠躬等提出的“哺乳动物垂体前叶神经-体液双重调节假说”提供了新的证据。
, 百拇医药
睾丸摘除后,垂体前叶中CGRP阳性神经纤维数量显著增多,表明垂体前叶内神经纤维可以对机体内分泌状态的剧烈变化作出积极地反应。免疫反应神经纤维的增加可能有两方面的原因:(1)神经纤维的生长,术后7d的时间已足够轴突生芽(sprouting)、分支。生长相关蛋白(GAP-43)与神经系统的发育、突触形成、可塑性以及神经再生有密切的关系;周围神经和中枢神经受损后,GAP-43的表达都增加,因此GAP-43可作为神经再生的标志。本研究室发现肾上腺摘除后4d,大鼠垂体前叶GAP-43阳性神经纤维数量明显增加,说明支配垂体前叶的神经纤维具有形态及功能可塑性,在机体内分泌状态改变的条件下,以发芽的方式可能调节前叶腺细胞的功能[14]。睾丸摘除后大鼠垂体前叶GAP-43阳性神经纤维也发生类似的变化(未发表资料)。(2)睾丸摘除前,大鼠垂体前叶中有一部分纤维内的CGRP含量较低,超出了免疫组织化学的灵敏度。睾丸摘除后,睾酮水平的变化影响这些神经纤维中CGRP的含量,可能使其中CGRP的含量有所升高,因而观察到垂体前叶中CGRP免疫反应神经纤维数量显著增加。综上所述,睾丸摘除后垂体前叶中CGRP免疫反应神经纤维的变化,可能既有神经纤维的生芽、分支、又有神经纤维中CGRP含量的增加。
, 百拇医药
图1 示正常对照组垂体前叶CGRP免疫反应神经纤维,膨体型纤维分布较稀疏。标尺示20μm
图2 睾丸摘除组垂体前叶CGRP免疫反应神经纤维,膨体型纤维分布较图1中显著稠密。标尺示40μm
图3 睾丸摘除组垂体前叶中CGRP阳性神经纤维,右下方粗纤维上发出许多迂曲盘绕的细纤维。标尺示20μm
图4 为图3的放大。油镜示粗纤维上较大的膨体及由粗纤维发出的许多细纤维,纤维分支处膨体较大。标尺示10μm
Fig.1 CGRP-immunoreactive nerve fibers in the anterior pituitary of the normal rat,the varicose fibers were distributed sparsely.Bar=20μm
, 百拇医药
Fig.2 CGRP-immunoreactive nerve fibers in the orchiectomized rat anterior pituitary,note that the varicose fibers were more densely distributed than that in Fig.1. Bar=40μm
Fig.3 CGRP-immunoreactive nerve fibers in the orchidectomized rat anterior pituitary,note that many thinner fibers were sent out from the thicker fiber(right bottom).Bar=20μm
Fig.4 Part magnification of Fig.3 Oil immersion.Note that many thin fibers were sent out from the thick fiber and there were many large varicosities along the thick and thin fibers.Bar=10μm
, http://www.100md.com
Δ Institute of Neurosciences,The Fourth Military Medical University,Xi'an 710032,China
参考文献
[1]Ju G, Liu SJ. Substance P-immunoreactive nerve fibers in the pars distalis of the anterior pituitary of macaques. J Chem Neuroanat, 1989a,2(6):349
[2]Ju G, Liu SJ. Relationship of substance P-immunoreactive nerve fibers to somatotropes of the anterior pituitary in the monkey. J Neuroendocrinol, 1989b,1(3):397
, 百拇医药
[3]Ju G, Liu SJ. The relationship of substance P-immunoreactive nerve fibers to thyrotropes and corticotropes in the pars distalis of the anterior pituitary in the monkey. Neuroscience, 1989c,32(2):441
[4]Ju G, Liu SJ, Ma D. Calcitonin gene-related peptide- and substance P-like immunoreactive innervation of the anterior pituitary in the rat. Neuroscience, 1993,54(4):981
[5]Mikkelsen JD, Larsen PJ, Moller M, et al. Substance P in the median eminence and pituitary of the rat: demonstration of immunoreactive fibers and specific binding sites. Neuroendocrinology, 1989,50(1):100
, 百拇医药
[6]Rosenfeld MG, Mermod JJ, Amura SG, et al. Production of a novel neuropeptide encoded by the calcitonin gene via tissue-specific RNA processing. Nature,1983,304(5922):129
[7]Skofitsch G, Jacobowitz DM. Calcitonin gene-related peptide: detailed immunohistochemical distribution in the central nervous system. Peptides, 1985,6(4):721
[8]Ju G, Zhang X. An electronic microscopical study on calcitonin gene-related peptide immunoreactive innervation of the anterior pituitary in the dog. J Comp Neurol, 1992,326(1):101
, 百拇医药
[9]Ju G, Ma D, Fan SC. Response of calcitonin gene-related peptide-like immunoreactive nerve fibers of the anterior pitutary to adrenalectomy in the rat. Neuroendocrinology,1994,59(5):505
[10]Gon G, Giaid A, Steel JH, et al. Localization of immunoreactivity for calcitonin gene-related peptide in the rat anterior pituitary during ontogeny and gonadal steroid manipulations and detection of its messenger ribonucleic acid. Endocrinology, 1990,127(6):2618
, 百拇医药
[11]Herbison AE, Dye S. Perinatal and adult factors responsible for the sexually dimorphic calcitonin gene-related peptide-containing cell population in the rat preoptic area. Neuroscience, 1993,54(4):991
[12]Wimalawansa SJ, Emson PC, MacIntyre I. Regional distribution of calcitonin gene-related peptide and its specific binding sites in rats with particular reference to the nervous system. Neuroendocrinology, 1987,46(2):131
[13]Fahim A, Rettori V, MacCann SM. The role of calcitonin gene-related peptide in the control of growth hormone and prolactin release. Neuroendocrinology, 1990,51(6):668
[14]Lu CR, Meng FD, Benewitz LI, et al. Evidence for axonal sprouting in the anterior pituitary following adrenalectomy in the rat. J Endocrinology, 1995,147(1):161
收稿1998-05 修回1998-08, 百拇医药
单位:卢春蓉 孟繁东 王浩军 鞠躬(第四军医大学神经科学研究所,西安 710032)
关键词:垂体前叶;CGRP;神经纤维;睾丸摘除;大鼠
解剖学报990304 【摘要】 目的 探讨体内睾酮水平变化对大鼠垂体前叶CGRP免疫反应神经纤维的影响。 方法 成年雄性SD大鼠随机分为7个组:正常对照组(NOR);睾丸摘除组(OX);假手术组(SO);正常+丙酸睾丸素组(NOR+TP);睾丸摘除+丙酸睾丸素组(OX+TP);正常+油剂组(NOR+V);睾丸摘除+油剂组(OX+V)。采用免疫组织化学和图像分析方法观察大鼠垂体前叶中CGRP免疫反应神经纤维的变化。 结果 睾丸摘除7d后,大鼠垂体前叶CGRP免疫反应神经纤维发生了显著变化,表现为膨体型纤维较正常组稠密,分支增多,迂曲成片,盘绕成网,分布更加广泛,膨体数量显著增多;粗纤维较正常组粗,且发出许多迂曲盘绕的细纤维,膨体形态变大。t检验显示,OX组与NOR组垂体前叶内CGRP免疫反应神经纤维的平均面积密度差异显著,OX+V与NOR组差异显著。而SO组、NOR+TP组、OX+TP组、NOR+V组与NOR组比较,CGRP免疫反应神经纤维的平均面积密度无显著差异。与NOR组比较,NOR+TP组、OX+TP组中,垂体前叶CGRP免疫反应神经纤维的形态以细纤维为主。 结论 垂体前叶中CGRP免疫反应神经纤维对体内睾酮水平的剧烈变化能作出积极反应,从功能形态学上证明了这些神经纤维对前叶腺细胞的分泌可起一定的调节作用。
, http://www.100md.com
THE CHANGES OF CALCITONIN GENE-RELATED PEPTIDE
IMMUNOREACTIVE NERVE FIBERS IN THE ANTERIOR
PITUITARY OF THE RAT FOLLOWING ORCHIECTOMY
Lu Chunrong, Meng Fandong, Wang Haojun, Ju GongΔ
(Institute of Neurosciences,The Fourth Military Medical University, Xi'an)
【Abstract】 Objective The present study was aimed at investigating the effects of the changes of testosterone levels on the calcitonin gene-related peptide(CGRP)-immunoreactive nerve fibers in the anterior pituitary(AP) of the rat.Methods The adult male SD rats were randomly divided into 7 groups: (1)normal(NOR), (2)orchiectomy(OX), (3)shame operation(SO), (4)normal+testosterone propionate(NOR+TP), (5)orchiectomy+testosterone propionate(OX+TP), (6)normal+vehicle(NOR+V), (7)orchiectomy+vehicle(OX+V). Immunohistochemistry and image analysis were used to study the changes of CGRP-immunoreactive nerve fibers in the AP of the rat.Results Following orchiectomy 7d,the amount of CGRP-immunoreactive nerve fibers in the AP of the rat increased significantly, with the tortuous varicose fibers more densely distributed than that of NOR and the amount of varicosities increased significantly, while the thick fibers were more thicker than that of NOR,and many thin fibers were sent out from the thick fibers, and the varicosities were more larger. Student's t-test showed that the difference of the average area density of the CGRP-immunoreactive nerve fibers in the AP between OX and NOR was significant. The difference of that between OX+V and NOR was also significant. But the difference of that between SO,NOR+TP,OX+TP,NOR+V and NOR was insignificant, whereas the appearance of the CGRP-immunoreactive nerve fibers of NOR+TP and OX+TP was different from that of NOR, with the former two had more thin fibers and less thick fibers.Conclusion The results demonstrated that CGRP-immunoreactive nerve fibers in the AP of the rat could respond actively to the violent change of circulating TP, suggesting that CGRP-immunoreactive nerve fibers might regulate secretion of the AP gland cells.
, 百拇医药
【Key words】 Anterior pituitary; Calcitonin gene-related peptide; Nerve Fibers; Orchiectomy; Rat
近年来,在一些哺乳动物垂体前叶中陆续发现了一定数量的SP及CGRP免疫反应神经纤维的分布[1~7]。免疫电镜研究发现犬垂体前叶CGRP免疫反应神经纤维与各种腺细胞联系密切,并可与ACTH细胞、GH细胞形成典型的不对称的突触结构[8],推测该神经纤维可能对ACTH、GH的合成与分泌具有调节作用。功能形态学和激素替代实验表明,对大鼠行双侧肾上腺摘除术后,垂体前叶中的CGRP免疫反应神经纤维数量显著增多,说明CGRP免疫反应神经纤维对机体的内分泌状态的变化产生了积极的反应[9],提示CGRP免疫反应神经纤维可能对垂体前叶的分泌发挥一定的调节作用。本研究观察了睾丸摘除后,大鼠体内雄性激素水平剧烈变化时,大鼠垂体前叶中CGRP免疫反应神经纤维的变化。
, 百拇医药
材料和方法
1. 动物
成年雄性SD大鼠56只,体重200~230g,由第四军医大学动物实验研究中心提供。随机分为7个组,每组8只:(1)正常对照组(NOR),(2)双侧睾丸摘除组(OX),(3)假手术组(SO),(4)正常+丙酸睾丸素组(NOR+TP),(5)睾丸摘除+丙酸睾丸素组(OX+TP),(6)正常+油剂组(NOR+V),(7)睾丸摘除+油剂组(OX+V)。
丙酸睾丸素的给药量为每日每只130μg(皮下注射),溶于芝麻油(0.2ml/只),于睾丸摘除次日开始给药。正常给药时间与摘除组给药时间同步,给药时间为每日17:30~18:00。给油剂时间与给药时间同步。饲养条件为12h光照(8:30~20:30),12h黑暗。给予足够的食物和饮水。
2. 取材
, 百拇医药
睾丸摘除后7d取材,取材时间为上午9:00~11:00,经升主动脉灌注100ml生理盐水及新鲜配制的4%多聚甲醛PB溶液(0.1mol/L,pH7.4)500ml,4℃,1h后取出垂体,在4℃、4%多聚甲醛PB溶液中后固定2~4h,入20%蔗糖PB溶液中,4℃过夜。为保证所有动物均在同一时间固定,每组实验相差1d。
3. 免疫组织化学方法
恒冷箱连续矢状切片,片厚30μm ,贴片于经1%明胶+0.05%硫酸铬钾处理过的载玻片上;在空气中干燥15min后,入0.01mol/L的KPBS(pH7.4),经100%甲醇+0.3%H2O2封闭内源性过氧化物酶,KPBS浸洗3×5min,浸泡于0.3%Triton X-100中30min,切片入兔抗CGRP血清(1:4 000,美国Salk Institute,W.Vale博士惠赠),抗体稀释液中含1%BSA、0.3%Triton X-100、0.02%NaN3,4℃孵育48h。KPBS浸洗3×10min,入结合生物素的羊抗兔第二抗体中室温孵育2~4h(1∶200,ABC Kit,Sigma),KPBS浸洗3×10min,入ABC复合物(1:200)室温孵育2~4h。葡萄糖氧化酶-DAB-硫酸镍胺法显色。常规脱水透明,DPX封片。对照实验用KPBS代替第一抗体,结果为阴性。
, 百拇医药
4. 图像分析
对每套切片随机等距抽取10张切片。在抽取的每张切片中,在×20物镜下再等距随机抽取5个视野,在图像分析仪Quantimat 570C上,测视野中CGRP免疫反应神经纤维的相对面积密度。计算50个视野中CGRP阳性神经纤维的总平均面积密度,对各实验组和对照组的相对平均面积密度进行团体t检验。
根据体视学卡瓦列里原理,垂体前叶的体积以其截面积来估算,即对每套切片等距抽取10张切片,测切片上前叶的面积,以其面积总和估算其体积值。t检验显示各组垂体前叶的体积之间无明显差别(P>0.25)。
结果
与正常对照组比,睾丸摘除7d后,大鼠垂体前叶CGRP免疫反应神经纤维中,其膨体型细纤维及粗纤维均发生变化,表现为:(1)膨体型纤维较正常组稠密,分布更加广泛,分支增多,成片迂曲盘绕成网,与腺细胞关系密切(图1,2)。(2)粗纤维较正常组粗,且发出许多迂曲盘绕的细纤维,可见其上大小不等的膨体,纤维分支处膨体形态较大(图3,4)。假手术组、正常+油剂组与正常对照组比,纤维平均面积密度无明显差异。摘除+油剂组与摘除组之间纤维平均面积密度也无明显差异,各组间纤维平均面积密度见附表。
, 百拇医药
附表 睾丸摘除后各组动物垂体前叶CGRP免疫反应神经纤维平均面积密度
Table Sample averaged area density of the CGRP-
immunoreactive nerve fibers in the anterior pituitaries of the rats following orchiectomy 动物分组
groups
平均面积密度(
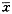 ±s)
±s)averaged area density
正常对照组(NOR)
, 百拇医药
0.0024±0.00055
假手术组(SO)
0.0024±0.00053
睾丸摘除组(OX)
0.0074±0.0011
正常+丙酸睾丸素组(NOR+TP)
0.0024±0.00055
睾丸摘除+丙酸睾丸素组(OX+TP)
0.0020±0.00023
正常+油剂组(NOR+V)
, 百拇医药
0.0026±0.00047
睾丸摘除+油剂组(OX+V)
0.0066±0.00058
与NOR组比:n=5,P<0.001 compared with NOR:n=5,**P<0.001
睾丸摘除+丙酸睾丸素组与正常对照组比较,纤维类型以膨体型细纤维为主,膨体较多。正常+丙酸睾丸素组与正常对照组比较,粗而直的纤维很少,大多为分布于垂体前叶内部中央,多弯曲、多膨体的细纤维。上述两组与正常组比较,纤维平均面积密度无明显差异。
讨论
有关睾酮与垂体前叶内CGRP合成的关系,前人作了一些初步的研究。如Gon等[10]发现大鼠垂体前叶内促性腺激素细胞合成、释放CGRP,当雄性大鼠去势后,垂体前叶CGRP阳性细胞数量显著增加,补充睾丸酮则能反转这种效应。该实验结果提示体内睾酮水平可能调控垂体前叶内促性腺激素细胞CGRP的表达,或者说在某种程度上睾酮可能抑制垂体前叶CGRP的表达。
, http://www.100md.com
除了垂体前叶内促性腺激素细胞能合成、释放CGRP之外,在正中隆起处还分布许多CGRP阳性神经纤维终末。这些CGRP纤维可能起源于下丘脑室周核,此部位均含有CGRP阳性细胞体[7]。雌雄鼠的视前区CGRP阳性细胞群存在显著性分化,雌鼠的内侧视前核中的CGRP阳性细胞比雄鼠中高20倍,而睾酮对于这种性分化是必需的。睾酮也对成年雄鼠的内侧视前核的CGRP阳性细胞发挥一种持续性的、潜在的抑制影响[11],有关这种抑制作用的机制尚不清楚。
CGRP能否在正中隆起处释放,通过垂体门脉系统,以类似下丘脑促垂体因子的方式作用于垂体,目前还不清楚。尽管有人未能在大鼠垂体前叶发现CGRP的受体,而后续研究报道大鼠垂体前叶有CGRP的特异结合位点。[12]。由于垂体前叶细胞能合成、释放CGRP,CGRP也可能从正中隆起通过垂体门脉系统到达垂体前叶,CGRP对垂体功能的作用即引起人们的关注。业已有一些研究认为CGRP可影响在体或离体下垂体前叶的分泌活动,如较低浓度的CGRP抑制离散培养的垂体前叶细胞分泌GH,而较高浓度的CGRP则刺激GH、PRL释放[13]。在支配垂体前叶的神经纤维中含有CGRP,CGRP可以调控垂体前叶的激素分泌,但CGRP阳性神经纤维在其中发挥什么作用,有待进一步研究。
, http://www.100md.com
本研究结果显示,睾丸摘除7d后,大鼠垂体前叶中CGRP阳性神经纤维数量显著增加,纤维变粗,纤维分支增多,膨体形态变大,数目增多。若在睾丸摘除后每日补充一定量的睾酮则能逆转这种变化,说明前叶中CGRP阳性神经纤维对体内睾酮水平的剧烈变化能作出反应。睾酮可作用于垂体和下丘脑水平,通过负反馈调节促性腺激素的分泌。睾丸摘除后,垂体前叶中FSH和LH含量及释放均增加。本实验结果说明,睾酮也反馈作用于支配垂体前叶的CGRP阳性神经元,影响其神经纤维中CGRP的含量。提示前叶中CGRP阳性神经纤维可能调节促性腺激素的分泌。
正常大鼠每日注射一定量的睾酮,导致体内睾酮水平升高时,与正常对照组比较,垂体前叶CGRP阳性神经纤维的平均面积密度一致,但两者神经纤维的形态分布却有较大的差异。推测可能的原因为:垂体前叶CGRP阳性神经纤维对体内睾酮水平降低或升高的反应程度不同;体内高水平睾酮可能影响了CGRP由粗纤维向细纤维的转运。
本实验结果与肾上腺摘除后大鼠垂体前叶中CGRP纤维的变化情况较相似[9],提示垂体前叶中CGRP阳性神经纤维可能对垂体前叶的功能发挥某些调控作用,从而为鞠躬等提出的“哺乳动物垂体前叶神经-体液双重调节假说”提供了新的证据。
, 百拇医药
睾丸摘除后,垂体前叶中CGRP阳性神经纤维数量显著增多,表明垂体前叶内神经纤维可以对机体内分泌状态的剧烈变化作出积极地反应。免疫反应神经纤维的增加可能有两方面的原因:(1)神经纤维的生长,术后7d的时间已足够轴突生芽(sprouting)、分支。生长相关蛋白(GAP-43)与神经系统的发育、突触形成、可塑性以及神经再生有密切的关系;周围神经和中枢神经受损后,GAP-43的表达都增加,因此GAP-43可作为神经再生的标志。本研究室发现肾上腺摘除后4d,大鼠垂体前叶GAP-43阳性神经纤维数量明显增加,说明支配垂体前叶的神经纤维具有形态及功能可塑性,在机体内分泌状态改变的条件下,以发芽的方式可能调节前叶腺细胞的功能[14]。睾丸摘除后大鼠垂体前叶GAP-43阳性神经纤维也发生类似的变化(未发表资料)。(2)睾丸摘除前,大鼠垂体前叶中有一部分纤维内的CGRP含量较低,超出了免疫组织化学的灵敏度。睾丸摘除后,睾酮水平的变化影响这些神经纤维中CGRP的含量,可能使其中CGRP的含量有所升高,因而观察到垂体前叶中CGRP免疫反应神经纤维数量显著增加。综上所述,睾丸摘除后垂体前叶中CGRP免疫反应神经纤维的变化,可能既有神经纤维的生芽、分支、又有神经纤维中CGRP含量的增加。

, 百拇医药
图1 示正常对照组垂体前叶CGRP免疫反应神经纤维,膨体型纤维分布较稀疏。标尺示20μm
图2 睾丸摘除组垂体前叶CGRP免疫反应神经纤维,膨体型纤维分布较图1中显著稠密。标尺示40μm
图3 睾丸摘除组垂体前叶中CGRP阳性神经纤维,右下方粗纤维上发出许多迂曲盘绕的细纤维。标尺示20μm
图4 为图3的放大。油镜示粗纤维上较大的膨体及由粗纤维发出的许多细纤维,纤维分支处膨体较大。标尺示10μm
Fig.1 CGRP-immunoreactive nerve fibers in the anterior pituitary of the normal rat,the varicose fibers were distributed sparsely.Bar=20μm
, 百拇医药
Fig.2 CGRP-immunoreactive nerve fibers in the orchiectomized rat anterior pituitary,note that the varicose fibers were more densely distributed than that in Fig.1. Bar=40μm
Fig.3 CGRP-immunoreactive nerve fibers in the orchidectomized rat anterior pituitary,note that many thinner fibers were sent out from the thicker fiber(right bottom).Bar=20μm
Fig.4 Part magnification of Fig.3 Oil immersion.Note that many thin fibers were sent out from the thick fiber and there were many large varicosities along the thick and thin fibers.Bar=10μm
, http://www.100md.com
Δ Institute of Neurosciences,The Fourth Military Medical University,Xi'an 710032,China
参考文献
[1]Ju G, Liu SJ. Substance P-immunoreactive nerve fibers in the pars distalis of the anterior pituitary of macaques. J Chem Neuroanat, 1989a,2(6):349
[2]Ju G, Liu SJ. Relationship of substance P-immunoreactive nerve fibers to somatotropes of the anterior pituitary in the monkey. J Neuroendocrinol, 1989b,1(3):397
, 百拇医药
[3]Ju G, Liu SJ. The relationship of substance P-immunoreactive nerve fibers to thyrotropes and corticotropes in the pars distalis of the anterior pituitary in the monkey. Neuroscience, 1989c,32(2):441
[4]Ju G, Liu SJ, Ma D. Calcitonin gene-related peptide- and substance P-like immunoreactive innervation of the anterior pituitary in the rat. Neuroscience, 1993,54(4):981
[5]Mikkelsen JD, Larsen PJ, Moller M, et al. Substance P in the median eminence and pituitary of the rat: demonstration of immunoreactive fibers and specific binding sites. Neuroendocrinology, 1989,50(1):100
, 百拇医药
[6]Rosenfeld MG, Mermod JJ, Amura SG, et al. Production of a novel neuropeptide encoded by the calcitonin gene via tissue-specific RNA processing. Nature,1983,304(5922):129
[7]Skofitsch G, Jacobowitz DM. Calcitonin gene-related peptide: detailed immunohistochemical distribution in the central nervous system. Peptides, 1985,6(4):721
[8]Ju G, Zhang X. An electronic microscopical study on calcitonin gene-related peptide immunoreactive innervation of the anterior pituitary in the dog. J Comp Neurol, 1992,326(1):101
, 百拇医药
[9]Ju G, Ma D, Fan SC. Response of calcitonin gene-related peptide-like immunoreactive nerve fibers of the anterior pitutary to adrenalectomy in the rat. Neuroendocrinology,1994,59(5):505
[10]Gon G, Giaid A, Steel JH, et al. Localization of immunoreactivity for calcitonin gene-related peptide in the rat anterior pituitary during ontogeny and gonadal steroid manipulations and detection of its messenger ribonucleic acid. Endocrinology, 1990,127(6):2618
, 百拇医药
[11]Herbison AE, Dye S. Perinatal and adult factors responsible for the sexually dimorphic calcitonin gene-related peptide-containing cell population in the rat preoptic area. Neuroscience, 1993,54(4):991
[12]Wimalawansa SJ, Emson PC, MacIntyre I. Regional distribution of calcitonin gene-related peptide and its specific binding sites in rats with particular reference to the nervous system. Neuroendocrinology, 1987,46(2):131
[13]Fahim A, Rettori V, MacCann SM. The role of calcitonin gene-related peptide in the control of growth hormone and prolactin release. Neuroendocrinology, 1990,51(6):668
[14]Lu CR, Meng FD, Benewitz LI, et al. Evidence for axonal sprouting in the anterior pituitary following adrenalectomy in the rat. J Endocrinology, 1995,147(1):161
收稿1998-05 修回1998-08, 百拇医药