心肌收缩蛋白基因表达、左室压及收缩力的近日节律
作者:王正荣 王 玲 万朝敏 Germaine Cornelissne,Inder Anand,Franz Halberg.
单位:王正荣.成都华西医科大学,成都 610047
关键词:近日节律;基因表达;心肌收缩;收缩能力
航天医学与医学工程990601摘要:目的 许多心血管变量存在着近日节律,心肌收缩反应及收缩蛋白基因表达是否存在着相应的周期性改变是值得深入研究。方法 在24h内采用直接在大白鼠左心室内插入左心导管记录左室压(LVP)和左室压力微分最大值(dp/dtmax)以及检测比较心肌细胞的α-MHC基因表达改变。结果 LVP(P<0.001)、dp/dtmax(P<0.001)和α-MHC(P<0.01)的变化存在着近日节律。通过比较三者近日节律振幅显示LVP的振幅最大,dp/dtmax次之,α-MHC基因表达的节律振幅再次之,表明心肌收缩力的近日节律的变化是由心肌细胞内在和外在作用的结果。结论 α-MHC基因表达水平的近日变化是决定着心肌收缩功能的近日节律的因素之一。
, http://www.100md.com
中图分类号:R331.31 文献标识码:A 文章编号:1002-0837(1999)06-0391-06
Circadian Rhythm of Gene Expression of Myocardial
Contractile Protein,Left Ventricular Pressure
and Contractility*
WANG Zheng-rong1,WANG Ling1,WAN Chao-min1,Germaine Cornelissen2,Inder Anand3,Franz Halberg2
, http://www.100md.com
(1.Biomedical Engineering Department, West China University of Medic al Sciences, Chengdu, 610041, P.R. China;2. Chronobiology Laboratories , University of Minnesota, Minneapolis, MN 55455, USA;3. Cardiology Section, VA Medical Center, Minneapolis, MN 55417,USA)
Abstract: Objective A number of cardiovascular variables exhibit a circ adian rhythm. Whethe r myocardial contractile response and gene expression of the contractile protein also show changes with a similar period was here investigated. Method Circadi an variabilities in the left ventricular developed pressure (LVP) and contractil ity (LV dp/dt max) were measured in 24 Sprague-Dawley r ats by directly left ve ntricular catheterizing and compared with changes in the gene expression of α- myosin heavy chain (α-MHC) in myocytes obtained from the same animals by dot b lottin g analysis. Results A circadian rhythm was seen in the variabili ty of LVP (P<0.001), LV dp/dt max (P<0.001) and the bio chemically measured expression of the α- MHC gene (P<0.01). As compared to the amplitude of the rhythm i n α-MHC gene exp ression, the amplitude of the contractility rhythm was large (P< 0.01) and the ci rcadian amplitude of the LVP(P<0.001) was the largest, represent ing perhaps a co mposite of intracardiac plus any extracardiac contributions. Conclusion One of factors determing the circadian rhythm of myocardial contractile function is α -MHC gene expression level.
, http://www.100md.com
Key words:circadian rhythm;gene expression;myocardial contracti on;contractility
Clinical and experimental studies have established that cardiovascular variables like blood pressure, heart rate, cardiac output and peripheral resistance exhib it spontaneous circadian variation in humans and other mammals[1~5]. Th e geneti c aspects of circadian variation are documented by its persistence in isolation from society[6]. Like higher frequencies, it is neither societally nor pacing i nduced[7].These investigations, including studies of twins[8,9] , do not diffe rentiate whether the circadian rhythm relates to the nervous, hormonal or metabo lic systems[10] or is intrinsic to cardiac muscle. Spoor and Jackson [11] demon strated a circadian rhythm in the sensitivity of isolated rat atria to acetylcho line. Goshima[12] found very high frequency ultradian variation in the contraction of cultured heart muscle cells.
, http://www.100md.com
We have reported in a single murine myocardial cell and in two sets of grouped c ells in culture for several days, variations in myocyte beating with anticipated about-daily, half-daily and half-weekly components, which could not be separ ated from trends in view of the brevity of the series[13]. Features of a presum ably bult-in spectrum of multifrequency rhythms, chaotic-appearing changes and tren ds are part of a broad organized time structure, the chronome[14]. Beca use myoc ytes in culture undergo rapid fibroblast transformation, it is difficult to be c ertain whether changes in the observed rhythm are related to circadian variation of the myocytes or are the result of fibroblast transformation. In humans, the timing of myocardial infarction, cardiac rhythm disturbances and other morbidit y as well as mortality have been shown to have definite cyclic patterns[1 5]. Wh ether such disturbances are influenced by variability intrinsic to myocytes rema ins to be determined.
, 百拇医药
Myocardial contractile function is influenced by the state of its contractile pr oteins, myosin and actin. An important component of the mammalian heart's contr actile protein is myosin heavy chian (MHC), which exists in three distinct isofo rms, V1, V2 and V3. The V1 and V3 isoforms are homodimers of the polypeptides en coded by α-MHC and β-MHC, and the V2 isoform is a heterodimer, consisting of α-M HC and β-MHC. The relative amounts of the three isoforms vary depending upon t he developmental stage and disease states. Under usual conditions, α-MHC (or V1) i s the predominant isoform in the adult rat. Hearts with a greater α-MHC gene e xp ression demonstrate a higher contractile response than hearts with a predominant β-MHC isoform.
, 百拇医药
In this study we tested the hypothesis that the rat heart undergoes a circadian variability in global myocardial contractility and that the latter is associated with similar circadian changes in the expression of contractile proteins.
Method
12 male and 12 female Sprague-Dawley rats, weighing 216±12g (SE), were stud ied.All animals were housed in an experimental animal laboratory under natural conditions of light alternating with darkness. Animals were randomly divided into 6 groups, each containing 2 m ale and 2 female rats and these sub-groups of 4 animals were studied at six test times, 4 hours apart (at 00:00, 04:00, 08:00, 12:00, 16:00 and 20:00, local tim e). After intraperitoneal administration of pentobarbital (30 mg/kg), the abdome n was opened and a polythene LV catheter (id 0.2 mm) was inserted into the animal's LV through the d iaphragm. The left ventricular pressure (LVP) and LV dp/dt were recorded using a pressure transducer (Statham P23Db) and a physiological polygraph system (Optic al Electronic, Japan). Hearts were then removed, atrial and the right ventricle discarded, and the LV was cleared of blood with saline solution and immediately sto red in a freezer at -80℃. For the analysis of α-MHC gene expression, total RN A w as prepared by the single-step method[16]. Briefly, LV tissue from eac h heart w as homogenized in a Teflon homogenizer with a denaturing solution containing 4M guanidium thiocyanate. The homogenate was mixed sequentially with 2M sodium acet ate (pH4), phenol, and chloroform/isoamyl alcohol. The resulting mixture was cen trifuged at 10000xg, yielding an upper aqueous phase containing total RNA. The total RNA content was measured for absorbent optical density with 260 nm UV-vis ible recording spectrophotometer (UV-280, Shimadzu, Japan).
, 百拇医药
The oligonucleotide probe of α-MHC was used to test for the α-MHC gene expre ssio n. The sequence of the probe was α-MHC, 5'-TTGTGGGATAGCAACAGCGA-3'. The oligon uc leotide probe was labeled with Digoxigenin-11-dUTP/dATP at the 3'-tailing, using the Digoxigenin labeling kit (S@CPolymer GmbH, Germany) as described in the Dig oxigenin labeling manual. 60 μg of total RNA were dropped through a nylon membr an e and fixed at 80℃ for 30 minutes. The membrane was prehybridized in a prehybri dizing solution 6.0 ml (5xSS, 0.1% N-Lauroy larcosine, 0.02% SDS and 1% Block ing Reagent, pH7. 0) at 58℃ for 3 hours, then was hybridized in hybridizing solution (prehybridiz ing solution 1.5 ml+20 ng/μl α-MHC Digoxigenin probe 40 μl) at 58℃ for 20 ho urs. A fter hybridization, the membrane was washed in 2xSSC for 10min, and then further washed in 0.1xSSC for 15 min at room temperature. Thereafter, the membrane was placed into washing buffer (20mM NaHPO4, 1% SDS, 1 mM EDTA) for 5 min, and colo r was developed in checking buffer (washing buffer-anti-Dig-Ap) 37℃ for 2 ho urs . After color was developed, the membrane was washed in water and dried at 80℃ for 2 min;then the optical absorbent density was measured in the hybridized dot with an optical density scanner (Shimadzu, Japan) to quantify α-MHC.
, 百拇医药
All time series were analyzed by the single cosinor method and the results thus obtained were compared by parameter tests[17]. The cosinor serves first to test the hypothesis of a zero circadian amplitude. If this “no-rhythm" hypothesis is rejected, the cosinor method also provides point-and-interval estimates of par am eters. These characteristics are the rhythm-adjusted mean (MESOR), the extent of predictable change, estimated by the circadian double amplitude, and the acroph ase, a measure of timing of overall high values recurring in each cycle. These p arameters were computed on the basis of both the original values for an assessme nt as such and after being expressed as a percentage of the MESOR. The latter re lative values were a priority relied upon. First, they served for the comparison of different, albeit related variables, originally expressed in different units . Second, the dynamic parameters (amplitude and acrophase) were of major interes t. The acrophase measures the timing of a rhythm as the delay from local midnigh t of the peak in the cosine curve best approximating all data; it is given in de grees, with 0° set to local midnight, and 360° equated to 24 hours. Hence, 1 h our=15° (360÷24=15), and 1°=4 minutes (60÷15=4).
, 百拇医药
Result
The LVP, LV dp/dt max, and α-MHC data on individual am inal are shown in Table 1. All variables investigated, LVP, myocardial contracility and contractil e protein gene expression, were high at night, and low during the day. Table 2 s hows the circadian characteristics of the three variables examined. A circadian rhythm is demonstrated time-microscopically (P<0.01) and also t ime-macroscopical ly (Fig.1). The relative circadian changes in LVP are the largest, ex hibiting a circadian double amplitude which is much larger than the change in ge ne expression (24.8 vs. 9.0% of the 24-hour mean value, Table 2). The correspond ing extent of predictable circadian rhythmic change of contractility, as gauged by the double amplitude, is intermediate (17.7%). The acrophases are very cl ose to each other and all occur during the dark span, that is the active span of the nocturnal rat (an acrophase of 35° corresponds to 02:20 and one of 52° to 03:28). Fig.1 Difference in circadian amplitude of α-MHC gene expression, left ventr icular pressure and LV dp/dt max. Timepoint means(SEM ar e given for data express ed as a percentage of respective 24-hour mean values (4 rats contributed data a t each timepoint). Circadian stage effect validated by 1-way analysis of varianc e in each case: LVP: F=19.852,P<0.001; LV dp/dt max: F=8.591, P<0.001; α-MHC gene expression (AD): F=4.446, P=0 .008
Fig.1 Difference in circadian amplitude of α-MHC gene expression, left ventr icular pressure and LV dp/dt max. Timepoint means(SEM ar e given for data express ed as a percentage of respective 24-hour mean values (4 rats contributed data a t each timepoint). Circadian stage effect validated by 1-way analysis of varianc e in each case: LVP: F=19.852,P<0.001; LV dp/dt max: F=8.591, P<0.001; α-MHC gene expression (AD): F=4.446, P=0 .008
, 百拇医药
The circadian characteristics of the three variables expressed as a percenta ge o f the 24-hour mean value are shown in Table 3. In the face of marked differences observed for the relative circadian amplitude, all acrophases are very similar, occurring within 1.2 hours of each other (P>0.20). This lack of a statistically significant difference in acrophase is important, in keeping with the close syn chronization shown in Table 2. Had the very small numerical difference in phase been validated, namely a delay of the acrophase (rather than a lead) in α-MHC g ene expression versus the pressure variables, this finding would have been contr ary to what was anticipated, namely a lead, assuming that gene exp ression has a most direct and hence a first b earing on the contractile property of the myocardial cells. The results on acrop hase are not at variance with this assumption, even if the main conclusion is a very tight synchronization of the changes in the three variables.
, http://www.100md.com
Table 1 Original values and timepoint averages of left ventricular pressure (LVP),myocardial contractility (LV dp/dt max) and gene expression of contractile protein (α-MHC) of Sprague-Dawley rats* variable(unit)
time (clock-hour)
00:00
04:00
08:00
12:00
16:00
, 百拇医药
20:00
LVP(mmHg)
122
125
108
95
92
105
119
118
112
98
94
, http://www.100md.com
112
115
122
96
102
96
98
116
117
103
105
95
106
, http://www.100md.com Mean±SE
118.0±1.6
120.5±1.8
104.8±3.4
100.0±2.2
94.3 ±0.9
105.3±2.9
LV dp/dt max (mmHg/s)
2250
2280
2180
1820
, 百拇医药
1810
2070
2270
2370
2250
1960
1850
2240
2180
2210
2070
1980
1860
, 百拇医药
2120
Mean±SE
2205±34
2260±42
2105±72
1955±50
1875±37
2103±54
α-MHC(A.D.)
516
522
526
, http://www.100md.com
472
462
506
518
506
518
528
459
521
505
532
487
491
, 百拇医药
478
476
527
517
505
512
468
508
Mean±SE
516.5±4.5
519.3±5.4
509.0±8.5
500.8±12.2
, 百拇医药
471 .3±6.4
502.8±9.5
*Studied under natural conditioins of light and darkness in August in Chengdu,Ch ina;A.D.=absorbent densityTable 2 Three murine circadian rhythms:1.in the expression of the myocardial contractile protein gene(α-MHC),2.in contractility (LV dp/dt max) and 3.in left ventricular pressure (LVP) variable examined
P
MESOR,M
, 百拇医药
double amplitude (95% CL)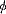 (95% CL)
(95% CL)
original values
% of M
α-MHC-AD(au)
0.002
503.3±3.5
39.7(19.3;60.1)
9.0(4.9;13.1)
-52°(-21;-83)
LV dp/dt max (mmHg)
, http://www.100md.com
<0.001
2083.8±19.7
369.6 (253.8;485.4)
17.7(12.2;23.3)
-37°(-19;-56)
LVP (mmHg)
<0.001
107.1±1.0
25.7(19.8;31.5)
24.8(19.3;30.4)
-35°(-22; -49)
, http://www.100md.com
*α-MHC=α-myosin heavy chain,gauged by AD=absorbent density of optical scanner when scanning the hybridized dot;au=arbitrary units;dp/dt max=maximal value of differential of LVP,P=P-value from zero-amplitude (no-rhythm) t est.Study un der conditions of natural illumination with artificial light turned on during sa mpling,on inbred,3-month old Sprague-Dawley rats;2 males and 2 females at each of 6 timepoints,4 hours apart.Acrophase in (negative) degrees,with 360°=24 h; acrophase reference:local 00:00=0°Table 3 Comparison of circadian characteristics in the contractile protein gene expression (α-MHC) of murine myocardial cells,in th eir contractility (dp/dt) and in left ventricular pressu re (LVP)* variables compared
, 百拇医药
comparison of:
amplitude,A
acrophase,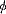
(A,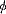 )
)
α-HMC-AD vs.dp/dt max
0.002
0.537
0.034
α-HMC-AD vs.LVP
, 百拇医药
<0.001
0.468
<0.001
dp/dt max vs.LVP
0.068
0.941
0.185
α-HMC-AD vs.dp/dt max vs.LVP
<0.001
0.827
0.001
*dp/dt max=maximal value of differential of LVP; AD=absorbent density of optical scanner when scanning the hybridized dot.Each variable expressed as a percentage of 24-hour mean value
, 百拇医药
Another finding is a difference in circadian amplitude visualized time-microscop ically in Table 2. The rhythm of α-MHC gene expression, albeit smallest in ext ent, is the most interesting new finding. This is apparently the first demonstra tion of the relative contribution of changes in intrinsic myocyte property to circadian changes in contractility and to LVP. These changes in myocyte α-MHC gene exp ress ion are likely to be amplified by many other circadian rhythmic extracardiac fac tors before affecting the overall circadian varibility of the circulation. Thus, it is not surprising that the circadian amplitudes of both contractility and le ft ventricular pressure are statistically significantly larger than that of the rhythm in gene expression (LVP:P<0.001; LV dp/dt max: P<0.01). A borderline sta tistically significant difference between the circadian amplitudes of left ventr icular pressure and contractility (P=0.068) may be accounted for by the fact that a number of hormonal, neural and metabolic factors, in addition to intrinsic m yocardial contractility, contribute to changes in cardiac output and peripheral resistance.
, 百拇医药
Discussion
In this study, we have demonstrated a circadian rhythmic pattern in myocardial c ontractility with a similar cicadian pattern in the gene expression of the α-m yo sin heavy chain. Similar circadian rhythms in gene expression of extracardiac pr oteins have previously been reported. The mRNA for rat liver serine dehydratase, a gluconeogenic enzyme, exhibits a circadian rhythm[18] with a maximum at the onset of darkness rather than during the middle of the dark span, as seen here w ith α-MHC. The expression of the GLUT5 gene in the intestine of rats and rabbi ts also showed a circadian rhythm[19] with a twelve-fold increase of val ues at th e end of the light span, again with a timing different from that found in this s tudy. A “10-100-fold rhythm" is reported for the mRNA of arylalkylamine N-acet yl transferase activity which coordinates rhythmic melatonin synthesis[20] . In Dro sophila, the genes “timeless" and “peroid" have been reported to collaborate to promote cycles of gene expression composing a circadian pacemaker. Autonomous cycling of mPer mRNA in the mouse suprachiasmatic nuclei has also been shown in environmental conditions of 12 hours of light alternating with 12 hours of darkn ess, as well as in continuous darkness.
, 百拇医药
The endogenous nature of the heartbeat has been known for long time. The Aztecs knew that the heart removed form the body can continue to beat. Recent studies using cinematography have revealed that mammalian myocytes in culture do not beat rand omly but have an endogenous time structure (chronome) with several components.Based on above studies, it again documents that whether mammals or a single cell , their behavior or function has their own intrinsic time structure; our study f urther proves that biological rhythm comes from the body or cell's own intrinsic time structure through the test combing molecular level with total body.
, 百拇医药
In our study, circadian rhythms of LVP, dp/dt max and α-MHC gene expression leve l are documented, however, the amplitude of LVP is the highest, dp/d t middle, and α-MHC gene expression level low. This result indicates that the circadian vari at ion of α-MHC gene expression is one of the reasons which cause myocardial cont ractile circadian rhythm,but not the only factor. In the body, myocardial contractile fu nction is regulated by neural and hormonal systems, those systems also show circadian v a riation. Therefore, circadian amplitude of α-MHC gene expression is smaller th an LVP and dp/dt max, since some hormonal and adrengergic nervous circadian variations effect on myocardium. In this study, since myocardial contractility is affected by hor monal and neural factors, the acrophase of α-MHC gene expression shows later t han that of LVP and dp/dt, but no statistical significan ce (P>0.1).
, 百拇医药
Summarizing above, myocardial contractile function and α-MHC gene expression i n rats show circadian rhythm, all the parameters are higher at night, lower during the day. The circadian variation of α-MHC gene expression is a basic factor re sulting in the circadian variation of myocardial contractile function.
*Foundation item:supported by National Nature Science Foundation of China(394701 93)
References
, 百拇医药
[1] Reinberg A, Smolensky MH. Biological rhythms and medicine. Cellular, meta bolic, physiopathologic, and pharmacologic aspects[M]. New York: Springer, 19 83:305
[2] Halberg F, Conelissen G, Halberg E et al. Chronobiology of human blood pressure[C]. 4th ed. Minneapolis: Medtronic Con tinuing Medical Education Seminars, 1988:242
[3] Huikuri HV, Kessler KM, Terracall E et al .Reproducibility and circadian rhythm of heart rate variability in healthy su b jects[J]. Am J Cardiol,1990,65:391~393
, 百拇医药
[4] Wang Z, Wang L, Zhang L et al.Circadian r e lations among cardiovascular variables of young adults[J]. Chronobiologia,1992 ,19:111~120
[5] Otsuka K, Cornelissen G, Halberg F. Circadian rhythmic fra ctal scaling of hea rtrate variability in health and coronary artery disease[J]. Clinical Cardiolo gy,1997,20:631~638
[6] Halberg F, Cornelissen G, Montalbini M et al.The biologic half-week (circasemise ptan ) and Kp: evolutionary and practical implications of magnetic field disturbances . Keynot #113, 2nd World Congress of Cellular and Molecular Biology, Ottawa, Can ada, September 3-7, 1996[J]. Cell Molec Biol,1996,42(Suppl.): S81~S82
, http://www.100md.com
[7] Castellanos A, Fuenmayor AJ, Huikuri H et al.D ynamics of atrioventricular nodal conduction ratios of reverse alternating Wenck ebach periods[J]. Am J Cardiol,1989,64:1047~1049
[8] Halberg F. Quo vadis basic and clinical chronobiology: Pro mise for health maintenance[J]. Am J Anat,1983,168:543~594
[9] Hanson BR, halberg F, Tuna N et al. Rhythmonetry reveals heritability of circadian characteritics of heart rat of human twins reared apart[J]. Cardiologia,1984,29:267~282
, 百拇医药
[10] Descovich GC, Montalbetti N, Kühl JFW et al. Age and catecholamine rhythms[J]. Chronobiologia, 1974,1:163~171
[11] Spoor RP, Jackson DB. Circadian rhythms: variation in sensitivity of isolated rat atria to acetylcholine[J]. Sciences,1966,154:782
[12] Goshima K. Rhythmic and arrhythmic contraction of cultured heart muscle cells. In: Suda M, Hayaishi O, Nakagawa H, eds. Biological Rhythms and th eir Central Mechanisms. Naito Foundation Symposium[M]. Amsterdam: Elsevier/ No rth-Holland Biomedical Press, 1979:67~81
, 百拇医药
[13] Han HW, Shao DL, Wu JY et al.Chronobio logic approach no beat-to-beat variations of cultured murine myocardial cells [J]. Cell Bioph ysics,1991,18:217~229
[14] Cornélissen G, Halberg F. Broadly pertinent chronobiology method s quantify phosphate dynamics (chronome) in blood and urine[J]. Clin Chem,1992,38:329~3 33
[15] Cornélissen G, Tamura K, Tarquini B et al.Differences in some circadia n patterns of cardiac arrhythmia, myocardial infarction and other adverse vascul ar events[J]. Chronobiologia,1994,21:79~88
, 百拇医药
[16] Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidi um thiocyanate-phenyl-chloroform extraction[J]. Anal Biochem,1987,162:156~1 59
[17] Bingham C, Arbogast B, Cornélissen Guillaume G et al.Inferent ial statistical methods for estimating and comparing cosinor parameters[J]. Ch ronobiologia,1982,9:397~439
[18] Ogawa H, Kawamats S. Periportal expression at the serine dehydrat ese gene in rat liver[J]. Histochemical J,1995,27:380~387
, http://www.100md.com
[19] Castello A, Guma A, Sevilla L et al.Re gulation of GLUT5 gene expression in rat intestinal mucosa: regional distributio n, circadian rhythm, perinatal development and effect of diabetes[J]. Biochemi cal J,1995,309:271~277
[20] Baler R, Covington S, Klein DC. The rat arylalkylamine N-acetylt ransferase gene promoter: cAMP activation via a cAMP-responisve element-CCAAT complex[J]. J Biol Chem,1997,272:6979~6985
Received date:1998-12-29, http://www.100md.com
单位:王正荣.成都华西医科大学,成都 610047
关键词:近日节律;基因表达;心肌收缩;收缩能力
航天医学与医学工程990601摘要:目的 许多心血管变量存在着近日节律,心肌收缩反应及收缩蛋白基因表达是否存在着相应的周期性改变是值得深入研究。方法 在24h内采用直接在大白鼠左心室内插入左心导管记录左室压(LVP)和左室压力微分最大值(dp/dtmax)以及检测比较心肌细胞的α-MHC基因表达改变。结果 LVP(P<0.001)、dp/dtmax(P<0.001)和α-MHC(P<0.01)的变化存在着近日节律。通过比较三者近日节律振幅显示LVP的振幅最大,dp/dtmax次之,α-MHC基因表达的节律振幅再次之,表明心肌收缩力的近日节律的变化是由心肌细胞内在和外在作用的结果。结论 α-MHC基因表达水平的近日变化是决定着心肌收缩功能的近日节律的因素之一。
, http://www.100md.com
中图分类号:R331.31 文献标识码:A 文章编号:1002-0837(1999)06-0391-06
Circadian Rhythm of Gene Expression of Myocardial
Contractile Protein,Left Ventricular Pressure
and Contractility*
WANG Zheng-rong1,WANG Ling1,WAN Chao-min1,Germaine Cornelissen2,Inder Anand3,Franz Halberg2
, http://www.100md.com
(1.Biomedical Engineering Department, West China University of Medic al Sciences, Chengdu, 610041, P.R. China;2. Chronobiology Laboratories , University of Minnesota, Minneapolis, MN 55455, USA;3. Cardiology Section, VA Medical Center, Minneapolis, MN 55417,USA)
Abstract: Objective A number of cardiovascular variables exhibit a circ adian rhythm. Whethe r myocardial contractile response and gene expression of the contractile protein also show changes with a similar period was here investigated. Method Circadi an variabilities in the left ventricular developed pressure (LVP) and contractil ity (LV dp/dt max) were measured in 24 Sprague-Dawley r ats by directly left ve ntricular catheterizing and compared with changes in the gene expression of α- myosin heavy chain (α-MHC) in myocytes obtained from the same animals by dot b lottin g analysis. Results A circadian rhythm was seen in the variabili ty of LVP (P<0.001), LV dp/dt max (P<0.001) and the bio chemically measured expression of the α- MHC gene (P<0.01). As compared to the amplitude of the rhythm i n α-MHC gene exp ression, the amplitude of the contractility rhythm was large (P< 0.01) and the ci rcadian amplitude of the LVP(P<0.001) was the largest, represent ing perhaps a co mposite of intracardiac plus any extracardiac contributions. Conclusion One of factors determing the circadian rhythm of myocardial contractile function is α -MHC gene expression level.
, http://www.100md.com
Key words:circadian rhythm;gene expression;myocardial contracti on;contractility
Clinical and experimental studies have established that cardiovascular variables like blood pressure, heart rate, cardiac output and peripheral resistance exhib it spontaneous circadian variation in humans and other mammals[1~5]. Th e geneti c aspects of circadian variation are documented by its persistence in isolation from society[6]. Like higher frequencies, it is neither societally nor pacing i nduced[7].These investigations, including studies of twins[8,9] , do not diffe rentiate whether the circadian rhythm relates to the nervous, hormonal or metabo lic systems[10] or is intrinsic to cardiac muscle. Spoor and Jackson [11] demon strated a circadian rhythm in the sensitivity of isolated rat atria to acetylcho line. Goshima[12] found very high frequency ultradian variation in the contraction of cultured heart muscle cells.
, http://www.100md.com
We have reported in a single murine myocardial cell and in two sets of grouped c ells in culture for several days, variations in myocyte beating with anticipated about-daily, half-daily and half-weekly components, which could not be separ ated from trends in view of the brevity of the series[13]. Features of a presum ably bult-in spectrum of multifrequency rhythms, chaotic-appearing changes and tren ds are part of a broad organized time structure, the chronome[14]. Beca use myoc ytes in culture undergo rapid fibroblast transformation, it is difficult to be c ertain whether changes in the observed rhythm are related to circadian variation of the myocytes or are the result of fibroblast transformation. In humans, the timing of myocardial infarction, cardiac rhythm disturbances and other morbidit y as well as mortality have been shown to have definite cyclic patterns[1 5]. Wh ether such disturbances are influenced by variability intrinsic to myocytes rema ins to be determined.
, 百拇医药
Myocardial contractile function is influenced by the state of its contractile pr oteins, myosin and actin. An important component of the mammalian heart's contr actile protein is myosin heavy chian (MHC), which exists in three distinct isofo rms, V1, V2 and V3. The V1 and V3 isoforms are homodimers of the polypeptides en coded by α-MHC and β-MHC, and the V2 isoform is a heterodimer, consisting of α-M HC and β-MHC. The relative amounts of the three isoforms vary depending upon t he developmental stage and disease states. Under usual conditions, α-MHC (or V1) i s the predominant isoform in the adult rat. Hearts with a greater α-MHC gene e xp ression demonstrate a higher contractile response than hearts with a predominant β-MHC isoform.
, 百拇医药
In this study we tested the hypothesis that the rat heart undergoes a circadian variability in global myocardial contractility and that the latter is associated with similar circadian changes in the expression of contractile proteins.
Method
12 male and 12 female Sprague-Dawley rats, weighing 216±12g (SE), were stud ied.All animals were housed in an experimental animal laboratory under natural conditions of light alternating with darkness. Animals were randomly divided into 6 groups, each containing 2 m ale and 2 female rats and these sub-groups of 4 animals were studied at six test times, 4 hours apart (at 00:00, 04:00, 08:00, 12:00, 16:00 and 20:00, local tim e). After intraperitoneal administration of pentobarbital (30 mg/kg), the abdome n was opened and a polythene LV catheter (id 0.2 mm) was inserted into the animal's LV through the d iaphragm. The left ventricular pressure (LVP) and LV dp/dt were recorded using a pressure transducer (Statham P23Db) and a physiological polygraph system (Optic al Electronic, Japan). Hearts were then removed, atrial and the right ventricle discarded, and the LV was cleared of blood with saline solution and immediately sto red in a freezer at -80℃. For the analysis of α-MHC gene expression, total RN A w as prepared by the single-step method[16]. Briefly, LV tissue from eac h heart w as homogenized in a Teflon homogenizer with a denaturing solution containing 4M guanidium thiocyanate. The homogenate was mixed sequentially with 2M sodium acet ate (pH4), phenol, and chloroform/isoamyl alcohol. The resulting mixture was cen trifuged at 10000xg, yielding an upper aqueous phase containing total RNA. The total RNA content was measured for absorbent optical density with 260 nm UV-vis ible recording spectrophotometer (UV-280, Shimadzu, Japan).
, 百拇医药
The oligonucleotide probe of α-MHC was used to test for the α-MHC gene expre ssio n. The sequence of the probe was α-MHC, 5'-TTGTGGGATAGCAACAGCGA-3'. The oligon uc leotide probe was labeled with Digoxigenin-11-dUTP/dATP at the 3'-tailing, using the Digoxigenin labeling kit (S@CPolymer GmbH, Germany) as described in the Dig oxigenin labeling manual. 60 μg of total RNA were dropped through a nylon membr an e and fixed at 80℃ for 30 minutes. The membrane was prehybridized in a prehybri dizing solution 6.0 ml (5xSS, 0.1% N-Lauroy larcosine, 0.02% SDS and 1% Block ing Reagent, pH7. 0) at 58℃ for 3 hours, then was hybridized in hybridizing solution (prehybridiz ing solution 1.5 ml+20 ng/μl α-MHC Digoxigenin probe 40 μl) at 58℃ for 20 ho urs. A fter hybridization, the membrane was washed in 2xSSC for 10min, and then further washed in 0.1xSSC for 15 min at room temperature. Thereafter, the membrane was placed into washing buffer (20mM NaHPO4, 1% SDS, 1 mM EDTA) for 5 min, and colo r was developed in checking buffer (washing buffer-anti-Dig-Ap) 37℃ for 2 ho urs . After color was developed, the membrane was washed in water and dried at 80℃ for 2 min;then the optical absorbent density was measured in the hybridized dot with an optical density scanner (Shimadzu, Japan) to quantify α-MHC.
, 百拇医药
All time series were analyzed by the single cosinor method and the results thus obtained were compared by parameter tests[17]. The cosinor serves first to test the hypothesis of a zero circadian amplitude. If this “no-rhythm" hypothesis is rejected, the cosinor method also provides point-and-interval estimates of par am eters. These characteristics are the rhythm-adjusted mean (MESOR), the extent of predictable change, estimated by the circadian double amplitude, and the acroph ase, a measure of timing of overall high values recurring in each cycle. These p arameters were computed on the basis of both the original values for an assessme nt as such and after being expressed as a percentage of the MESOR. The latter re lative values were a priority relied upon. First, they served for the comparison of different, albeit related variables, originally expressed in different units . Second, the dynamic parameters (amplitude and acrophase) were of major interes t. The acrophase measures the timing of a rhythm as the delay from local midnigh t of the peak in the cosine curve best approximating all data; it is given in de grees, with 0° set to local midnight, and 360° equated to 24 hours. Hence, 1 h our=15° (360÷24=15), and 1°=4 minutes (60÷15=4).
, 百拇医药
Result
The LVP, LV dp/dt max, and α-MHC data on individual am inal are shown in Table 1. All variables investigated, LVP, myocardial contracility and contractil e protein gene expression, were high at night, and low during the day. Table 2 s hows the circadian characteristics of the three variables examined. A circadian rhythm is demonstrated time-microscopically (P<0.01) and also t ime-macroscopical ly (Fig.1). The relative circadian changes in LVP are the largest, ex hibiting a circadian double amplitude which is much larger than the change in ge ne expression (24.8 vs. 9.0% of the 24-hour mean value, Table 2). The correspond ing extent of predictable circadian rhythmic change of contractility, as gauged by the double amplitude, is intermediate (17.7%). The acrophases are very cl ose to each other and all occur during the dark span, that is the active span of the nocturnal rat (an acrophase of 35° corresponds to 02:20 and one of 52° to 03:28).
 Fig.1 Difference in circadian amplitude of α-MHC gene expression, left ventr icular pressure and LV dp/dt max. Timepoint means(SEM ar e given for data express ed as a percentage of respective 24-hour mean values (4 rats contributed data a t each timepoint). Circadian stage effect validated by 1-way analysis of varianc e in each case: LVP: F=19.852,P<0.001; LV dp/dt max: F=8.591, P<0.001; α-MHC gene expression (AD): F=4.446, P=0 .008
Fig.1 Difference in circadian amplitude of α-MHC gene expression, left ventr icular pressure and LV dp/dt max. Timepoint means(SEM ar e given for data express ed as a percentage of respective 24-hour mean values (4 rats contributed data a t each timepoint). Circadian stage effect validated by 1-way analysis of varianc e in each case: LVP: F=19.852,P<0.001; LV dp/dt max: F=8.591, P<0.001; α-MHC gene expression (AD): F=4.446, P=0 .008, 百拇医药
The circadian characteristics of the three variables expressed as a percenta ge o f the 24-hour mean value are shown in Table 3. In the face of marked differences observed for the relative circadian amplitude, all acrophases are very similar, occurring within 1.2 hours of each other (P>0.20). This lack of a statistically significant difference in acrophase is important, in keeping with the close syn chronization shown in Table 2. Had the very small numerical difference in phase been validated, namely a delay of the acrophase (rather than a lead) in α-MHC g ene expression versus the pressure variables, this finding would have been contr ary to what was anticipated, namely a lead, assuming that gene exp ression has a most direct and hence a first b earing on the contractile property of the myocardial cells. The results on acrop hase are not at variance with this assumption, even if the main conclusion is a very tight synchronization of the changes in the three variables.
, http://www.100md.com
Table 1 Original values and timepoint averages of left ventricular pressure (LVP),myocardial contractility (LV dp/dt max) and gene expression of contractile protein (α-MHC) of Sprague-Dawley rats* variable(unit)
time (clock-hour)
00:00
04:00
08:00
12:00
16:00
, 百拇医药
20:00
LVP(mmHg)
122
125
108
95
92
105
119
118
112
98
94
, http://www.100md.com
112
115
122
96
102
96
98
116
117
103
105
95
106
, http://www.100md.com Mean±SE
118.0±1.6
120.5±1.8
104.8±3.4
100.0±2.2
94.3 ±0.9
105.3±2.9
LV dp/dt max (mmHg/s)
2250
2280
2180
1820
, 百拇医药
1810
2070
2270
2370
2250
1960
1850
2240
2180
2210
2070
1980
1860
, 百拇医药
2120
Mean±SE
2205±34
2260±42
2105±72
1955±50
1875±37
2103±54
α-MHC(A.D.)
516
522
526
, http://www.100md.com
472
462
506
518
506
518
528
459
521
505
532
487
491
, 百拇医药
478
476
527
517
505
512
468
508
Mean±SE
516.5±4.5
519.3±5.4
509.0±8.5
500.8±12.2
, 百拇医药
471 .3±6.4
502.8±9.5
*Studied under natural conditioins of light and darkness in August in Chengdu,Ch ina;A.D.=absorbent densityTable 2 Three murine circadian rhythms:1.in the expression of the myocardial contractile protein gene(α-MHC),2.in contractility (LV dp/dt max) and 3.in left ventricular pressure (LVP) variable examined
P
MESOR,M
, 百拇医药
double amplitude (95% CL)
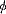 (95% CL)
(95% CL)original values
% of M
α-MHC-AD(au)
0.002
503.3±3.5
39.7(19.3;60.1)
9.0(4.9;13.1)
-52°(-21;-83)
LV dp/dt max (mmHg)
, http://www.100md.com
<0.001
2083.8±19.7
369.6 (253.8;485.4)
17.7(12.2;23.3)
-37°(-19;-56)
LVP (mmHg)
<0.001
107.1±1.0
25.7(19.8;31.5)
24.8(19.3;30.4)
-35°(-22; -49)
, http://www.100md.com
*α-MHC=α-myosin heavy chain,gauged by AD=absorbent density of optical scanner when scanning the hybridized dot;au=arbitrary units;dp/dt max=maximal value of differential of LVP,P=P-value from zero-amplitude (no-rhythm) t est.Study un der conditions of natural illumination with artificial light turned on during sa mpling,on inbred,3-month old Sprague-Dawley rats;2 males and 2 females at each of 6 timepoints,4 hours apart.Acrophase in (negative) degrees,with 360°=24 h; acrophase reference:local 00:00=0°Table 3 Comparison of circadian characteristics in the contractile protein gene expression (α-MHC) of murine myocardial cells,in th eir contractility (dp/dt) and in left ventricular pressu re (LVP)* variables compared
, 百拇医药
comparison of:
amplitude,A
acrophase,
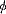
(A,
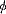 )
)α-HMC-AD vs.dp/dt max
0.002
0.537
0.034
α-HMC-AD vs.LVP
, 百拇医药
<0.001
0.468
<0.001
dp/dt max vs.LVP
0.068
0.941
0.185
α-HMC-AD vs.dp/dt max vs.LVP
<0.001
0.827
0.001
*dp/dt max=maximal value of differential of LVP; AD=absorbent density of optical scanner when scanning the hybridized dot.Each variable expressed as a percentage of 24-hour mean value
, 百拇医药
Another finding is a difference in circadian amplitude visualized time-microscop ically in Table 2. The rhythm of α-MHC gene expression, albeit smallest in ext ent, is the most interesting new finding. This is apparently the first demonstra tion of the relative contribution of changes in intrinsic myocyte property to circadian changes in contractility and to LVP. These changes in myocyte α-MHC gene exp ress ion are likely to be amplified by many other circadian rhythmic extracardiac fac tors before affecting the overall circadian varibility of the circulation. Thus, it is not surprising that the circadian amplitudes of both contractility and le ft ventricular pressure are statistically significantly larger than that of the rhythm in gene expression (LVP:P<0.001; LV dp/dt max: P<0.01). A borderline sta tistically significant difference between the circadian amplitudes of left ventr icular pressure and contractility (P=0.068) may be accounted for by the fact that a number of hormonal, neural and metabolic factors, in addition to intrinsic m yocardial contractility, contribute to changes in cardiac output and peripheral resistance.
, 百拇医药
Discussion
In this study, we have demonstrated a circadian rhythmic pattern in myocardial c ontractility with a similar cicadian pattern in the gene expression of the α-m yo sin heavy chain. Similar circadian rhythms in gene expression of extracardiac pr oteins have previously been reported. The mRNA for rat liver serine dehydratase, a gluconeogenic enzyme, exhibits a circadian rhythm[18] with a maximum at the onset of darkness rather than during the middle of the dark span, as seen here w ith α-MHC. The expression of the GLUT5 gene in the intestine of rats and rabbi ts also showed a circadian rhythm[19] with a twelve-fold increase of val ues at th e end of the light span, again with a timing different from that found in this s tudy. A “10-100-fold rhythm" is reported for the mRNA of arylalkylamine N-acet yl transferase activity which coordinates rhythmic melatonin synthesis[20] . In Dro sophila, the genes “timeless" and “peroid" have been reported to collaborate to promote cycles of gene expression composing a circadian pacemaker. Autonomous cycling of mPer mRNA in the mouse suprachiasmatic nuclei has also been shown in environmental conditions of 12 hours of light alternating with 12 hours of darkn ess, as well as in continuous darkness.
, 百拇医药
The endogenous nature of the heartbeat has been known for long time. The Aztecs knew that the heart removed form the body can continue to beat. Recent studies using cinematography have revealed that mammalian myocytes in culture do not beat rand omly but have an endogenous time structure (chronome) with several components.Based on above studies, it again documents that whether mammals or a single cell , their behavior or function has their own intrinsic time structure; our study f urther proves that biological rhythm comes from the body or cell's own intrinsic time structure through the test combing molecular level with total body.
, 百拇医药
In our study, circadian rhythms of LVP, dp/dt max and α-MHC gene expression leve l are documented, however, the amplitude of LVP is the highest, dp/d t middle, and α-MHC gene expression level low. This result indicates that the circadian vari at ion of α-MHC gene expression is one of the reasons which cause myocardial cont ractile circadian rhythm,but not the only factor. In the body, myocardial contractile fu nction is regulated by neural and hormonal systems, those systems also show circadian v a riation. Therefore, circadian amplitude of α-MHC gene expression is smaller th an LVP and dp/dt max, since some hormonal and adrengergic nervous circadian variations effect on myocardium. In this study, since myocardial contractility is affected by hor monal and neural factors, the acrophase of α-MHC gene expression shows later t han that of LVP and dp/dt, but no statistical significan ce (P>0.1).
, 百拇医药
Summarizing above, myocardial contractile function and α-MHC gene expression i n rats show circadian rhythm, all the parameters are higher at night, lower during the day. The circadian variation of α-MHC gene expression is a basic factor re sulting in the circadian variation of myocardial contractile function.
*Foundation item:supported by National Nature Science Foundation of China(394701 93)
References
, 百拇医药
[1] Reinberg A, Smolensky MH. Biological rhythms and medicine. Cellular, meta bolic, physiopathologic, and pharmacologic aspects[M]. New York: Springer, 19 83:305
[2] Halberg F, Conelissen G, Halberg E et al. Chronobiology of human blood pressure[C]. 4th ed. Minneapolis: Medtronic Con tinuing Medical Education Seminars, 1988:242
[3] Huikuri HV, Kessler KM, Terracall E et al .Reproducibility and circadian rhythm of heart rate variability in healthy su b jects[J]. Am J Cardiol,1990,65:391~393
, 百拇医药
[4] Wang Z, Wang L, Zhang L et al.Circadian r e lations among cardiovascular variables of young adults[J]. Chronobiologia,1992 ,19:111~120
[5] Otsuka K, Cornelissen G, Halberg F. Circadian rhythmic fra ctal scaling of hea rtrate variability in health and coronary artery disease[J]. Clinical Cardiolo gy,1997,20:631~638
[6] Halberg F, Cornelissen G, Montalbini M et al.The biologic half-week (circasemise ptan ) and Kp: evolutionary and practical implications of magnetic field disturbances . Keynot #113, 2nd World Congress of Cellular and Molecular Biology, Ottawa, Can ada, September 3-7, 1996[J]. Cell Molec Biol,1996,42(Suppl.): S81~S82
, http://www.100md.com
[7] Castellanos A, Fuenmayor AJ, Huikuri H et al.D ynamics of atrioventricular nodal conduction ratios of reverse alternating Wenck ebach periods[J]. Am J Cardiol,1989,64:1047~1049
[8] Halberg F. Quo vadis basic and clinical chronobiology: Pro mise for health maintenance[J]. Am J Anat,1983,168:543~594
[9] Hanson BR, halberg F, Tuna N et al. Rhythmonetry reveals heritability of circadian characteritics of heart rat of human twins reared apart[J]. Cardiologia,1984,29:267~282
, 百拇医药
[10] Descovich GC, Montalbetti N, Kühl JFW et al. Age and catecholamine rhythms[J]. Chronobiologia, 1974,1:163~171
[11] Spoor RP, Jackson DB. Circadian rhythms: variation in sensitivity of isolated rat atria to acetylcholine[J]. Sciences,1966,154:782
[12] Goshima K. Rhythmic and arrhythmic contraction of cultured heart muscle cells. In: Suda M, Hayaishi O, Nakagawa H, eds. Biological Rhythms and th eir Central Mechanisms. Naito Foundation Symposium[M]. Amsterdam: Elsevier/ No rth-Holland Biomedical Press, 1979:67~81
, 百拇医药
[13] Han HW, Shao DL, Wu JY et al.Chronobio logic approach no beat-to-beat variations of cultured murine myocardial cells [J]. Cell Bioph ysics,1991,18:217~229
[14] Cornélissen G, Halberg F. Broadly pertinent chronobiology method s quantify phosphate dynamics (chronome) in blood and urine[J]. Clin Chem,1992,38:329~3 33
[15] Cornélissen G, Tamura K, Tarquini B et al.Differences in some circadia n patterns of cardiac arrhythmia, myocardial infarction and other adverse vascul ar events[J]. Chronobiologia,1994,21:79~88
, 百拇医药
[16] Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidi um thiocyanate-phenyl-chloroform extraction[J]. Anal Biochem,1987,162:156~1 59
[17] Bingham C, Arbogast B, Cornélissen Guillaume G et al.Inferent ial statistical methods for estimating and comparing cosinor parameters[J]. Ch ronobiologia,1982,9:397~439
[18] Ogawa H, Kawamats S. Periportal expression at the serine dehydrat ese gene in rat liver[J]. Histochemical J,1995,27:380~387
, http://www.100md.com
[19] Castello A, Guma A, Sevilla L et al.Re gulation of GLUT5 gene expression in rat intestinal mucosa: regional distributio n, circadian rhythm, perinatal development and effect of diabetes[J]. Biochemi cal J,1995,309:271~277
[20] Baler R, Covington S, Klein DC. The rat arylalkylamine N-acetylt ransferase gene promoter: cAMP activation via a cAMP-responisve element-CCAAT complex[J]. J Biol Chem,1997,272:6979~6985
Received date:1998-12-29, http://www.100md.com