HCV E2和HBV preS融合蛋白真核表达载体的构建及在哺乳动物细胞中的表达
作者:谢 尧 陶其敏
单位:北京医科大学人民医院肝病研究所,北京 100044
关键词:肝炎病毒组;丙型;肝炎病毒;乙型;遗传载体;代谢;病毒包膜蛋白质类;代谢;蛋白质S;代谢
北京医科大学学报990111 摘 要 目的:构建丙型肝炎病毒(HCV)E2和乙型肝炎病毒(HBV)preS融合蛋白的真核表达载体。方法:用PCR方法扩增HBV preS和HCV E2蛋白基因,并将二者克隆到真核表达载体pcDNA3中,用脂质体转染试剂将其转入COS7细胞,通过免疫荧光检测细胞瞬时表达的融合蛋白。结果:E2-preS融合蛋白基因包括了全长的HBV preS和HCV E2蛋白基因,嵌合基因长度约1.6 kb。表达载体在COS7细胞表达出了E2-preS融合蛋白。结论:构建的E2-preS融合蛋白表达载体能在哺乳动物细胞中表达融合蛋白,为获得真核表达的融合蛋白及研究此蛋白的免疫原性打下基础。
, 百拇医药
中国图书资料分类法分类号 R512.6-332
Constructing the eukaryotic expression vector expressing HCV E2 protein combined with HBV preS protein and expressing the combined protein in mammalian cells
XIE Yao, TAO Qi-Min
(Institute of Hepatology, People's Hospital, Beijing Medical University,Beijing 100044)
MeSH Hepatitis C viruses Hepatitis B virus Genetic vectors/metab Viral envelope proteins/metab Protein S/metab
, http://www.100md.com
ABSTRACT Objective: To construct the eukaryotic expression vector which expresses the combined protein of HCV E2 and HBV preS proteins. Methods: HCV E2 and HBV preS genes were amplified with PCR and cloned into mammalian expression vector pcDNA3. The constructed vector was transfected into COS7 cells with lipofectin. The expressed E2-preS protein was detected by means of immunofluorescence. Results: The chimeric gene, which was about 1.6 kb, included entire HBV preS and HCV E2 gene. The cells transfected with the constructed vector expressed E2-preS protein successfully. Conclusion: The constructed vector containing chimeric gene of E2-preS protein can express E2-preS protein in mammalian cell. The success of constructing this vector laid the foundation for obtaining the combined protein and studying its immunogenicity.
, http://www.100md.com
(J Beijing Med Univ, 1999,31:38-40)
对丙型肝炎病毒(hepatitis C virus, HCV)包膜蛋白的研究显示,其E2蛋白的抗体对病毒具有中和活性[1,2],能阻断HCV对细胞的附着[3],但只有高滴度抗体机体才能完全免受HCV的攻击[4]。有报道,E2蛋白可诱导细胞免疫[5,6],而细胞免疫在预防或从HCV感染的恢复中可能起着重要作用。
由于乙型肝炎病毒(hepatitis B virus, HBV)的preS蛋白含有附加的T细胞和B细胞表位[7,8],HBV包膜蛋白与HIV的蛋白多肽结合,能诱导出抗此多肽的抗体[9]。为增加HCV E2蛋白的免疫原性,我们构建了E2-preS融合蛋白的真核表达载体,并在真核细胞表达了此融合蛋白,以期通过preS蛋白中的T细胞和B细胞表位增强HCV E2包膜蛋白的免疫原性。
, 百拇医药
1 材料与方法
1.1 目的基因的扩增
根据中国HBV-adr亚型的DNA序列设计引物,用PCR方法以HBV DNA阳性血清中提取的HBV DNA为模板扩增preS全长基因(包括preS1和preS2),在上、下游分别引入EcoRⅠ和XbaⅠ酶切位点,下游引入终止密码子。
上游引物为:
5′-CAGTCGAATTCATGGGAGGTTGGTCTTCC-3′
下游引物为:
5′-TACATTCTAGATTAGTTCGGTGCAGGGTCCC-3′
以本所克隆的中国HCV基因Ⅲ型丙肝病毒E2包膜蛋白基因为模板,扩增E2包膜蛋白基因,上、下游分别引入HindⅢ和EcoRⅠ酶切位点,上游引入起始密码子。
, http://www.100md.com
上游引物为:
5′-ATATAAAGCTTATGGCCGCCGGGGTTGAC-3′
下游引物为:
5′-TACATGAATTCCATCCACAAGCAGGCGC-3′
扩增产物经电泳回收。
1.2 真核表达载体的构建、纯化和DNA测序
以pcDNA3质粒为真核表达载体,将载体及preS基因片段分别用EcoRⅠ和XbaⅠ进行双酶切,电泳回收目的片段。用T4 DNA连接酶将二者连接,产物转化JM109细菌,经酶切鉴定获得含有preS基因的阳性克隆。以同样的方法将E2基因片段克隆于含有preS基因的质粒中,经酶切鉴定获得含有E2和preS基因的阳性克隆,命名为pcDNAE2-preS。用聚乙二醇沉淀法纯化DNA。DNA测序由赛百盛公司完成。
, 百拇医药
1.3 E2-preS蛋白在哺乳动物细胞中的表达
以COS7细胞为宿主细胞。转染DNA前1天,细胞传代于6孔板中,用脂质体转染试剂DOTAP(Boehringer-Mannheim公司)将质粒DNA转染细胞6 h,6 h后换新鲜培养基继续培养50 h。细胞经胰酶消化、PBS洗涤3次后,制成细胞悬液,滴于处理好的载玻片上,干燥后经丙酮固定、PBS洗涤,制成细胞滴片,用preS2单抗为一抗,FITC标记的羊抗鼠IgG为二抗作免疫荧光检测,以转染pcDNA3空载体的细胞为阴性对照。
2 结果
扩增的HBV preS基因约为520 bp,HCV E2基因约为1.1 kb。构建后的融合蛋白表达载体pcDNAE2-preS的结构见图1。用HindⅢ及XbaⅠ双酶切可切下约为1.6 kb的E2-preS基因片段及5.4 kb的空载体。用HindⅢ、 EcoRⅠ和XbaⅠ 3个内切酶切割可切下长度分别为1.1 kb、 520 bp及5.4 kb的E2、preS基因和空载体(图2)。DNA测序证实融合蛋白基因含有1 620个核苷酸,读码框架正确。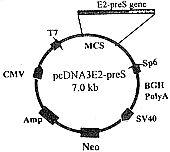
, 百拇医药
图1 E2-preS融合蛋白真核表达载体的结构
Figure 1 Construction of vector expressing E2-preS protein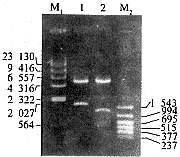
1,treated with restriction endonuclease HindⅢ and XbaⅠ; 2,treated with restriction endonuclease HindⅢ, EcoRⅠ and XbaⅠ; M,nucleotide marker(bp).
图2 pcDNAE2-preS质粒酶切鉴定的电泳图谱
Figure 2 The electrophoresis map of pcDNAE2-preS
, 百拇医药
digested with restriction endonuclease
在融合蛋白瞬时表达的免疫荧光检测中,转化了pcDNAE2-preS的细胞与preS2单抗孵育后,再加入FITC标记的二抗反应,荧光显微镜下可见细胞发出荧光,而转染pcDNA3空载体的细胞在同样条件下不发光,结果见图3。
A,cells, transfected with PCDNA3, incubated with preS2-specific monoclonal antibody were not luminous; B,PCDN3E2-pres transfected cell incubated with preS2-specific monoclonal antibody were luminous.
, http://www.100md.com 图3 COS7细胞瞬时表达preS和E2融合蛋白的免疫荧光检测
Figure 3 Immunofluorescence staining of the chimeric protein
of preS and E2 antigen transiently expressed in COS7 cells
3 讨论
虽然E2包膜蛋白抗体与HCV病毒的清除相关,并能中和HCV的感染活性,但在慢性HCV感染患者E2抗体的检测中却发现,有很大一部分患者有E2抗体[10],且此抗体的检出与HCV RNA的检出相关[11]。其原因可能为HCV病毒在体内的低复制[12],循环中HCV滴度很低,包膜蛋白的免疫原性弱,对机体不能产生足够的免疫刺激,从而无足够的E2抗体来清除HCV。而只有高滴度的E2抗体才能获得对HCV感染的完全性保护[4]。为此我们构建了E2-preS融合蛋白的真核表达载体,希望借助融合蛋白中preS的附加T细胞和B细胞表位,以增强E2蛋白的免疫原性。
, http://www.100md.com
融合蛋白基因包括了全长的HBV preS基因和全长的HCV E2包膜蛋白基因,DNA测序结果显示,融合蛋白基因含有1 620个核苷酸,读码框架正确,从而保证了表达产物的正确性。用preS2单抗对细胞表达的E2-preS蛋白进行免疫荧光检测,在显微镜下见pcDNAE2-preS转染的细胞发出荧光,而对照细胞不发光,说明转染融合蛋白表达载体的细胞有preS2蛋白的表达。因preS2基因处于E2蛋白及preS1基因的下游,preS2基因相当于报告基因,它的表达可以表明有E2和preS1蛋白的表达。由于瞬时表达的融合蛋白产量低,要进一步研究此蛋白的免疫原性,有待以后从稳定的细胞表达中获得更多的蛋白或进行基因免疫的研究。目前我们正在进行这方面的工作。
参考文献
1 Shimizu YK, Hijikata M, Lwamoto A. Neutralizing antibodies against hepatitis C virus and the emergence of neutralization escape mutant viruses. J Virol, 1994,68:1494-1500
, 百拇医药
2 Farci P, Harvry HJ, Wong DC. Prevention of hepatitis C virus infection in chimpanzees after antibody-mediated in vitro neutralization. Proc Natl Acad Sci USA, 1994,91:7792-7796
3 Zibert A, Schreier E, Roggendorf M. Antibody in human sera specific to hypervariable region 1 of hepatitis C virus can block viral attachment. Virology, 1995,208:653-661
4 Choo QL, Kuo G, Ralston R, et al. Vaccination of chimpanzees against infection by the hepatitis C virus. Proc Natl Acad Sci USA, 1994,91:1294-1298
, 百拇医药
5 Koziel MJ, Dudley D, Wong JT. Intrahepatic cytotoxic T lymphocytes specific for hepatitis C virus in persons with chronic hepatitis. J Immunol, 1992,149:3339-3344
6 Koziel MJ, Dudley D, Afdehl N. Hepatitis C virus-specific cytotoxic T lymphocytes recognize epitopes in the core and envelope regions of HCV. J Virol, 1993,67:7522-7528
7 Milich DR, Mclachlon A, Chisari FV, et al. Immune response to the preS1 region of the hepatitis B surface antigen(HBsAg): a preS1- specific T cell response can bypass nonresponsiveness to the preS2 and S regions of HBsAg. J Immunol, 1986,137:315-322
, 百拇医药
8 Shouval D, Ilan Y, Adler R. Improved immunogenicity in mice of a mammalian cell-derived recombinant hepatitis B vaccine as compared with conventional yeast-derived vaccines. Vaccine, 1994,12:1453-1459
9 Neurath AR, Strick N, Girard M. Hepatitis B virus surface antigen(HBsAg) as carrier for synthetic peptides having an attached hydrophobic tail. Mol Immunol, 1989,26:53-62
10 Lesniewski RR, Boardway KM, Casey JM. Hypervariable 5'-terminus of hepatitis C virus E2/NS1 encodes antigenically distinct variants. J Med Virol, 1993,40:150-156
, 百拇医药
11 Lesniewski R, Okasinski G, Carrick R. Antibody to hepatitis C virus second envelope (HCV-E2) glycoprotein: a new marker of HCV infection closely associated with viremia. J Med Virol, 1995,45:415-422
12 Prince AM, Brotman B, Huima T, et al. Immunity in hepatitis C infection. J Infect Dis, 1992,165:438-443
(1998-04-09收稿)
(本文编辑:景 霞 周传敬), http://www.100md.com
单位:北京医科大学人民医院肝病研究所,北京 100044
关键词:肝炎病毒组;丙型;肝炎病毒;乙型;遗传载体;代谢;病毒包膜蛋白质类;代谢;蛋白质S;代谢
北京医科大学学报990111 摘 要 目的:构建丙型肝炎病毒(HCV)E2和乙型肝炎病毒(HBV)preS融合蛋白的真核表达载体。方法:用PCR方法扩增HBV preS和HCV E2蛋白基因,并将二者克隆到真核表达载体pcDNA3中,用脂质体转染试剂将其转入COS7细胞,通过免疫荧光检测细胞瞬时表达的融合蛋白。结果:E2-preS融合蛋白基因包括了全长的HBV preS和HCV E2蛋白基因,嵌合基因长度约1.6 kb。表达载体在COS7细胞表达出了E2-preS融合蛋白。结论:构建的E2-preS融合蛋白表达载体能在哺乳动物细胞中表达融合蛋白,为获得真核表达的融合蛋白及研究此蛋白的免疫原性打下基础。
, 百拇医药
中国图书资料分类法分类号 R512.6-332
Constructing the eukaryotic expression vector expressing HCV E2 protein combined with HBV preS protein and expressing the combined protein in mammalian cells
XIE Yao, TAO Qi-Min
(Institute of Hepatology, People's Hospital, Beijing Medical University,Beijing 100044)
MeSH Hepatitis C viruses Hepatitis B virus Genetic vectors/metab Viral envelope proteins/metab Protein S/metab
, http://www.100md.com
ABSTRACT Objective: To construct the eukaryotic expression vector which expresses the combined protein of HCV E2 and HBV preS proteins. Methods: HCV E2 and HBV preS genes were amplified with PCR and cloned into mammalian expression vector pcDNA3. The constructed vector was transfected into COS7 cells with lipofectin. The expressed E2-preS protein was detected by means of immunofluorescence. Results: The chimeric gene, which was about 1.6 kb, included entire HBV preS and HCV E2 gene. The cells transfected with the constructed vector expressed E2-preS protein successfully. Conclusion: The constructed vector containing chimeric gene of E2-preS protein can express E2-preS protein in mammalian cell. The success of constructing this vector laid the foundation for obtaining the combined protein and studying its immunogenicity.
, http://www.100md.com
(J Beijing Med Univ, 1999,31:38-40)
对丙型肝炎病毒(hepatitis C virus, HCV)包膜蛋白的研究显示,其E2蛋白的抗体对病毒具有中和活性[1,2],能阻断HCV对细胞的附着[3],但只有高滴度抗体机体才能完全免受HCV的攻击[4]。有报道,E2蛋白可诱导细胞免疫[5,6],而细胞免疫在预防或从HCV感染的恢复中可能起着重要作用。
由于乙型肝炎病毒(hepatitis B virus, HBV)的preS蛋白含有附加的T细胞和B细胞表位[7,8],HBV包膜蛋白与HIV的蛋白多肽结合,能诱导出抗此多肽的抗体[9]。为增加HCV E2蛋白的免疫原性,我们构建了E2-preS融合蛋白的真核表达载体,并在真核细胞表达了此融合蛋白,以期通过preS蛋白中的T细胞和B细胞表位增强HCV E2包膜蛋白的免疫原性。
, 百拇医药
1 材料与方法
1.1 目的基因的扩增
根据中国HBV-adr亚型的DNA序列设计引物,用PCR方法以HBV DNA阳性血清中提取的HBV DNA为模板扩增preS全长基因(包括preS1和preS2),在上、下游分别引入EcoRⅠ和XbaⅠ酶切位点,下游引入终止密码子。
上游引物为:
5′-CAGTCGAATTCATGGGAGGTTGGTCTTCC-3′
下游引物为:
5′-TACATTCTAGATTAGTTCGGTGCAGGGTCCC-3′
以本所克隆的中国HCV基因Ⅲ型丙肝病毒E2包膜蛋白基因为模板,扩增E2包膜蛋白基因,上、下游分别引入HindⅢ和EcoRⅠ酶切位点,上游引入起始密码子。
, http://www.100md.com
上游引物为:
5′-ATATAAAGCTTATGGCCGCCGGGGTTGAC-3′
下游引物为:
5′-TACATGAATTCCATCCACAAGCAGGCGC-3′
扩增产物经电泳回收。
1.2 真核表达载体的构建、纯化和DNA测序
以pcDNA3质粒为真核表达载体,将载体及preS基因片段分别用EcoRⅠ和XbaⅠ进行双酶切,电泳回收目的片段。用T4 DNA连接酶将二者连接,产物转化JM109细菌,经酶切鉴定获得含有preS基因的阳性克隆。以同样的方法将E2基因片段克隆于含有preS基因的质粒中,经酶切鉴定获得含有E2和preS基因的阳性克隆,命名为pcDNAE2-preS。用聚乙二醇沉淀法纯化DNA。DNA测序由赛百盛公司完成。
, 百拇医药
1.3 E2-preS蛋白在哺乳动物细胞中的表达
以COS7细胞为宿主细胞。转染DNA前1天,细胞传代于6孔板中,用脂质体转染试剂DOTAP(Boehringer-Mannheim公司)将质粒DNA转染细胞6 h,6 h后换新鲜培养基继续培养50 h。细胞经胰酶消化、PBS洗涤3次后,制成细胞悬液,滴于处理好的载玻片上,干燥后经丙酮固定、PBS洗涤,制成细胞滴片,用preS2单抗为一抗,FITC标记的羊抗鼠IgG为二抗作免疫荧光检测,以转染pcDNA3空载体的细胞为阴性对照。
2 结果
扩增的HBV preS基因约为520 bp,HCV E2基因约为1.1 kb。构建后的融合蛋白表达载体pcDNAE2-preS的结构见图1。用HindⅢ及XbaⅠ双酶切可切下约为1.6 kb的E2-preS基因片段及5.4 kb的空载体。用HindⅢ、 EcoRⅠ和XbaⅠ 3个内切酶切割可切下长度分别为1.1 kb、 520 bp及5.4 kb的E2、preS基因和空载体(图2)。DNA测序证实融合蛋白基因含有1 620个核苷酸,读码框架正确。

, 百拇医药
图1 E2-preS融合蛋白真核表达载体的结构
Figure 1 Construction of vector expressing E2-preS protein

1,treated with restriction endonuclease HindⅢ and XbaⅠ; 2,treated with restriction endonuclease HindⅢ, EcoRⅠ and XbaⅠ; M,nucleotide marker(bp).
图2 pcDNAE2-preS质粒酶切鉴定的电泳图谱
Figure 2 The electrophoresis map of pcDNAE2-preS
, 百拇医药
digested with restriction endonuclease
在融合蛋白瞬时表达的免疫荧光检测中,转化了pcDNAE2-preS的细胞与preS2单抗孵育后,再加入FITC标记的二抗反应,荧光显微镜下可见细胞发出荧光,而转染pcDNA3空载体的细胞在同样条件下不发光,结果见图3。

A,cells, transfected with PCDNA3, incubated with preS2-specific monoclonal antibody were not luminous; B,PCDN3E2-pres transfected cell incubated with preS2-specific monoclonal antibody were luminous.
, http://www.100md.com 图3 COS7细胞瞬时表达preS和E2融合蛋白的免疫荧光检测
Figure 3 Immunofluorescence staining of the chimeric protein
of preS and E2 antigen transiently expressed in COS7 cells
3 讨论
虽然E2包膜蛋白抗体与HCV病毒的清除相关,并能中和HCV的感染活性,但在慢性HCV感染患者E2抗体的检测中却发现,有很大一部分患者有E2抗体[10],且此抗体的检出与HCV RNA的检出相关[11]。其原因可能为HCV病毒在体内的低复制[12],循环中HCV滴度很低,包膜蛋白的免疫原性弱,对机体不能产生足够的免疫刺激,从而无足够的E2抗体来清除HCV。而只有高滴度的E2抗体才能获得对HCV感染的完全性保护[4]。为此我们构建了E2-preS融合蛋白的真核表达载体,希望借助融合蛋白中preS的附加T细胞和B细胞表位,以增强E2蛋白的免疫原性。
, http://www.100md.com
融合蛋白基因包括了全长的HBV preS基因和全长的HCV E2包膜蛋白基因,DNA测序结果显示,融合蛋白基因含有1 620个核苷酸,读码框架正确,从而保证了表达产物的正确性。用preS2单抗对细胞表达的E2-preS蛋白进行免疫荧光检测,在显微镜下见pcDNAE2-preS转染的细胞发出荧光,而对照细胞不发光,说明转染融合蛋白表达载体的细胞有preS2蛋白的表达。因preS2基因处于E2蛋白及preS1基因的下游,preS2基因相当于报告基因,它的表达可以表明有E2和preS1蛋白的表达。由于瞬时表达的融合蛋白产量低,要进一步研究此蛋白的免疫原性,有待以后从稳定的细胞表达中获得更多的蛋白或进行基因免疫的研究。目前我们正在进行这方面的工作。
参考文献
1 Shimizu YK, Hijikata M, Lwamoto A. Neutralizing antibodies against hepatitis C virus and the emergence of neutralization escape mutant viruses. J Virol, 1994,68:1494-1500
, 百拇医药
2 Farci P, Harvry HJ, Wong DC. Prevention of hepatitis C virus infection in chimpanzees after antibody-mediated in vitro neutralization. Proc Natl Acad Sci USA, 1994,91:7792-7796
3 Zibert A, Schreier E, Roggendorf M. Antibody in human sera specific to hypervariable region 1 of hepatitis C virus can block viral attachment. Virology, 1995,208:653-661
4 Choo QL, Kuo G, Ralston R, et al. Vaccination of chimpanzees against infection by the hepatitis C virus. Proc Natl Acad Sci USA, 1994,91:1294-1298
, 百拇医药
5 Koziel MJ, Dudley D, Wong JT. Intrahepatic cytotoxic T lymphocytes specific for hepatitis C virus in persons with chronic hepatitis. J Immunol, 1992,149:3339-3344
6 Koziel MJ, Dudley D, Afdehl N. Hepatitis C virus-specific cytotoxic T lymphocytes recognize epitopes in the core and envelope regions of HCV. J Virol, 1993,67:7522-7528
7 Milich DR, Mclachlon A, Chisari FV, et al. Immune response to the preS1 region of the hepatitis B surface antigen(HBsAg): a preS1- specific T cell response can bypass nonresponsiveness to the preS2 and S regions of HBsAg. J Immunol, 1986,137:315-322
, 百拇医药
8 Shouval D, Ilan Y, Adler R. Improved immunogenicity in mice of a mammalian cell-derived recombinant hepatitis B vaccine as compared with conventional yeast-derived vaccines. Vaccine, 1994,12:1453-1459
9 Neurath AR, Strick N, Girard M. Hepatitis B virus surface antigen(HBsAg) as carrier for synthetic peptides having an attached hydrophobic tail. Mol Immunol, 1989,26:53-62
10 Lesniewski RR, Boardway KM, Casey JM. Hypervariable 5'-terminus of hepatitis C virus E2/NS1 encodes antigenically distinct variants. J Med Virol, 1993,40:150-156
, 百拇医药
11 Lesniewski R, Okasinski G, Carrick R. Antibody to hepatitis C virus second envelope (HCV-E2) glycoprotein: a new marker of HCV infection closely associated with viremia. J Med Virol, 1995,45:415-422
12 Prince AM, Brotman B, Huima T, et al. Immunity in hepatitis C infection. J Infect Dis, 1992,165:438-443
(1998-04-09收稿)
(本文编辑:景 霞 周传敬), http://www.100md.com