丹参酮ⅡA磺酸钠含量测定
 |
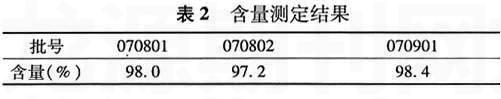 |
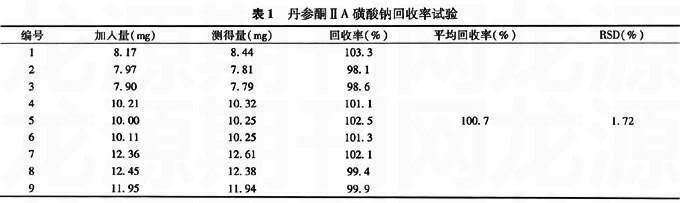
【摘要】 目的 建立丹参酮ⅡA磺酸钠原料药含量测定方法。方法 采用高效液相色谱法,以十八烷基硅烷键合硅胶为填充剂;以0.2%磷酸二氢钾溶液(用1.5 mol/L磷酸调节pH值至3.0)-甲醇(80∶20)为流动相;检测波长为271 nm;流速为1.0 ml/min;柱温为室温。结果 丹参酮ⅡA磺酸钠浓度在25.68~205.4μg/ml时,方法线性关系良好(r=0.999 9,n=5),进样精密度RSD为0.13%(n=5)。结论 此法简单,结果准确可靠,可用于丹参酮ⅡA磺酸钠原料药的质量控制。
【关键词】 丹参酮ⅡA磺酸钠;高效液相色谱法;含量测定
Content Determination of sulfotanshinone sodium
LIU Yong-qing.Troditional Drug Application Deoelopment Section,Liaoning Public Justic Management Cadre College,Liaoning 110163,China
【Abstract】 Objective To determine sulfotanshinone sodium in bulk drug.Methods HPLC was adopted,The column was Cl8,the detection wavelength was 271 nm and the flow rate was 1 ml/min.The chromatography was conducted at room temperature. Results There was a good linear relationship within25.68-205.4 μg/ml (r=0.9999 n=5).The RSD was 0.13%(n=5).Conclusion The method proposed for content determination of sulfotanshinone sodium andby HPLC is simple,accurate and can be used in quality control of sulfotanshinone sodium. ......
您现在查看是摘要页,全文长 4800 字符。